SWIMS News 09
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

BARB Establishment Survey Annual Data Report Volume 2: BBC Areas
BARB Establishment Survey Annual Data Report Volume 2: BBC Areas January 2011 to December 2011 BARB ESTABLISHMENT SURVEY OF TV HOMES Page 1 DATA PERIOD: ANNUAL January - December 2011 Contents Page Introduction 2 Annual Data Tables: Volume 2 - BBC areas BBC London 3 BBC South East 31 BBC Midlands 59 BBC East 87 BBC West 115 BBC South West 143 BBC South 171 BBC Yorkshire and Lincolnshire 199 BBC North East & Cumbria 227 BBC North West 255 BBC Scotland 283 BBC Ulster 311 BBC Wales 339 BBC Midlands West 367 BBC Midlands East 395 See also Volume 1 - Total Network and Appendices Volume 3 - ITV areas Introduction Page 2 This reports contains the weighted data results from the Establishment survey for the period January - December 2011 Data is presented at different levels Household Set Individual Accordingly; bases do vary. Appendices can be found in the Volume 1 report. These contain details of the survey objectives, sample design, response rates and standard definitions. A copy of the questionnaire is also included at the end of the report. BBC London Page 3 Contents Page Household Table 1.1: Social Grade 4 Table 1.2: Housewife Age 5 Table 1.3: Size of Household 6 Table 1.4: Presence of Children 7 Table 1.5: Number of TV sets in household 8 Table 1.6: Screen size 9 Table 1.7: Location of ANY set in household 10 Table 1.8: Recorders 11 Table 1.9: Other TV equipment 12 Table 1.10: Computers and Internet 13 Sets Table 1.11: Screen Size 14 Table 1.12: Location of set 15 Table 1.13: Recorders 16 Table 1.14: Other TV equipment 16 Table 1.15: Main -

Select Committee of Tynwald on the Television Licence Fee Report 2010/11
PP108/11 SELECT COMMITTEE OF TYNWALD ON THE TELEVISION LICENCE FEE REPORT 2010/11 REPORT OF THE SELECT COMMITTEE OF TYNWALD ON THE TELEVISION LICENCE FEE At the sitting of Tynwald Court on 18th November 2009 it was resolved - "That Tynwald appoints a Committee of three Members with powers to take written and oral evidence pursuant to sections 3 and 4 of the Tynwald Proceedings Act 1876, as amended, to investigate the feasibility and impact of withdrawal from or amendment of the agreement under which residents of the Isle of Man pay a television licence fee; and to report." The powers, privileges and immunities relating to the work of a committee of Tynwald are those conferred by sections 3 and 4 of the Tynwald Proceedings Act 1876, sections 1 to 4 of the Privileges of Tynwald (Publications) Act 1973 and sections 2 to 4 of the Tynwald Proceedings Act 1984. Mr G D Cregeen MHK (Malew & Santon) (Chairman) Mr D A Callister MLC Hon P A Gawne MHK (Rushen) Copies of this Report may be obtained from the Tynwald Library, Legislative Buildings, Finch Road, Douglas IM7 3PW (Tel 07624 685520, Fax 01624 685522) or may be consulted at www, ,tynwald.orgim All correspondence with regard to this Report should be addressed to the Clerk of Tynwald, Legislative Buildings, Finch Road, Douglas IMI 3PW TABLE OF CONTENTS 1. Introduction 1 2. The broadcasting landscape in the Isle of Man 4 Historical background 4 Legal framework 5 The requirement to pay the licence fee 5 Whether the licence fee is a UK tax 6 Licence fee collection and enforcement 7 Infrastructure for terrestrial broadcasting 10 Television 10 Radio: limitations of analogue transmission capability and extent of DAB coverage 13 3. -

The DRM Trial
Project Mayflower: The DRM Trial Final Report April 2009 1 Project Mayflower: The DRM Trial Trial Summary Introduction The BBC and its transmission provider, National Grid Wireless, have recently undertaken a trial of digital radio mondiale (DRM), a technology which allows digital broadcasting at frequencies lower than 30 MHz. The trial ran for a year from April 2007. The final report of the trial is made up of three separate documents: − this trial summary report, which provides some background and draws together the headline conclusions; − a final audience research report, which outlines the results of the research undertaken with an audience panel over the year1; and − a BBC R&D white paper, which provides the results and analysis of continuous unattended measuring and logging of the transmission2. Reflecting the way in which the trial was organised, each of these reports has been written by a different part of the team involved. The final audience research report has been written by the company employed to undertake the research – Leapfrog Research & Planning – with the assistance of BBC Marketing, Communications & Audiences. The technical note has been written by a member of the team who built the continuous logging network at BBC Research & Development and who was involved in developing the underlying DRM technology. 1 “Project Mayflower ‐ Digital Radio Mondiale (DRM) Trial: Final audience research summary report”, Daniel Amarasinghe (Leapfrog Planning & Research), Russell Chant (BBC MC&A); August 2008 2 BBC Research White Paper 174, “The Plymouth Digital Radio Mondiale (Drm) Trial: Long‐term Reception Results”, Andrew Murphy; February 2009 2 Background to the trial The digital radio mondiale (DRM) technology is an international standard which was designed to allow digital broadcasting at frequencies below 30 MHz, that is in the broadcasting bands which currently rely on AM transmission. -
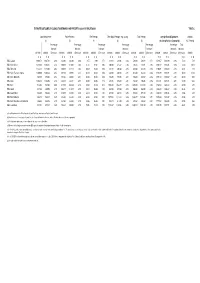
Data by BBC Region
ESTIMATED OUTCOME OF COUNCIL TAX DEMANDS AND PRECEPTS 2020/21 BY BBC REGION TABLE C Local Requirement Police Precepts Fire Precepts Other Major Precepts (e.g. County) Total Precepts Average Band D Equivalent of which: (1) (2) (3) (4) (5) (including Parish / Community) ASC Precept Percentage Percentage Percentage Percentage Percentage Percentage Total Increase / Increase / Increase / Increase / Increase / Increase / Increase / 2019/20 2020/21 (Decrease) 2019/20 2020/21 (Decrease) 2019/20 2020/21 (Decrease) 2019/20 2020/21 (Decrease) 2019/20 2020/21 (Decrease) 2019/20 2020/21 (Decrease) (Decrease) 2020/21 £ p £ p £ p £ p £ p £ p £ p £ p £ p £ p £ p £ p £ p £ p BBC London 1,008.73 1,047.23 3.8% 233.96 243.80 4.2% 8.75 8.90 1.7% 318.13 328.96 3.4% 560.84 581.66 3.7% 1,569.57 1,628.89 3.8% 59.32 17.21 BBC North West 1,256.82 1,303.25 3.7% 200.70 210.68 5.0% 45.45 46.36 2.0% 300.30 315.37 5.0% 546.45 572.41 4.8% 1,803.27 1,875.66 4.0% 72.39 20.17 BBC Yorkshire 1,133.13 1,172.56 3.5% 205.99 214.14 4.0% 69.32 70.69 2.0% 347.37 358.63 3.2% 622.68 643.46 3.3% 1,755.81 1,816.02 3.4% 60.21 14.12 BBC North East & Cumbria 1,260.69 1,308.38 3.8% 201.83 208.85 3.5% 63.18 64.42 2.0% 347.39 360.16 3.7% 612.40 633.43 3.4% 1,873.09 1,941.81 3.7% 68.72 21.42 BBC West Midlands 945.58 979.05 3.5% 191.24 200.68 4.9% 63.94 65.24 2.0% 534.36 556.65 4.2% 789.54 822.57 4.2% 1,735.12 1,801.62 3.8% 66.50 16.93 BBC West 1,104.14 1,149.05 4.1% 223.13 232.51 4.2% 62.20 63.25 1.7% 451.64 469.30 3.9% 736.97 765.06 3.8% 1,841.11 1,914.11 4.0% 73.00 19.44 BBC East 513.04 -

BBC Local Radio, Local News & Current Affairs Quantitative Research
BBC Local Radio, Local News & Current Affairs Quantitative Research August - September 2015 A report by ICM on behalf of the BBC Trust Creston House, 10 Great Pulteney Street, London W1F 9NB [email protected] | www.icmunlimited.com | +44 020 7845 8300 (UK) | +1 212 886 2234 (US) ICM Research Ltd. Registered in England No. 2571387. Registered Address: Creston House, 10 Great Pulteney Street, London W1F 9NB A part of Creston Unlimited BBC Trust Local Services Review, 2015 - Report Contents Executive summary .......................................................................................................................... 3 1. Background and methodology ................................................................................................. 6 1.1 Background ........................................................................................................................... 6 1.2 Methodology ......................................................................................................................... 6 1.3 Presentation and interpretation of the data ......................................................................... 7 2. BBC Local Radio ...................................................................................................................... 9 2.3 Types of local news and information consumed – unprompted ......................................... 11 2.4 Times of day BBC Local Radio is listened to, tenure with station and hours per week listened .......................................................................................................................................... -

Issue 164 Sept 2016
What is September 2016 this? It is a QR Code: get No. 164 a QR reader on a Nailsworth smartphone, scan this and it will take you to News our website! A free monthly community paper for the parish of Nailsworth, available in colour on our website www.nailsworth news.org.uk ee Centre Spread for details of our glorious SNailsworth Market. We are blessed to have such things on our doorstep with local people selling local produce. Take a peek! Nailsworth has a new Ambassador! “I’m Elsie Heslop. I am 13 years old and I am a pupil at Sir William Romney’s school in Tetbury. This year I have again been lucky enough to be selected as the school’s ambassador for Forest Green Rovers. My role as an Ambassador is to help promote the local football club both within the school and also the local community. I like to do reports on matches and the Club and inform my friends about what is going on. I was an ambassador last season and was lucky enough to be voted runner up for ‘Secondary School Ambassador of the Year’ and as a result of that, I have been lucky to be chosen again to carry on my role throughout this season. My reports will be about various things at the Club and emembering young lives lost too regular updates about FGR and ‘Rsoon’ is the theme behind a stunning what they’re doing. Hopefully sculpture recently unveiled in Cossack Square. you will enjoy my reports!” Elsie will be writing every two Please turn to page 4 for details of the months or so for the NN and we poignant, yet inspirational and beautiful are delighted welcome her on tribute to Jinny-Mae Cook. -

BBC Radio Post-1967
1967 1968 1969 1970 1971 1972 1973 1974 1975 1976 1977 1978 1979 1980 1981 1982 1983 1984 1985 1986 1987 1988 1989 1990 1991 1992 1993 1994 1995 1996 1997 1998 1999 2000 2001 2002 2003 2004 2005 2006 2007 2008 2009 2010 2011 2012 2013 2014 2015 2016 2017 2018 2019 2020 2021 Operated by BBC Radio 1 BBC Radio 1 Dance BBC Radio 1 relax BBC 1Xtra BBC Radio 1Xtra BBC Radio 2 BBC Radio 3 National BBC Radio 4 BBC Radio BBC 7 BBC Radio 7 BBC Radio 4 Extra BBC Radio 5 BBC Radio 5 Live BBC Radio Five Live BBC Radio 5 Live BBC Radio Five Live Sports Extra BBC Radio 5 Live Sports Extra BBC 6 Music BBC Radio 6 Music BBC Asian Network BBC World Service International BBC Radio Cymru BBC Radio Cymru Mwy BBC Radio Cymru 2 Wales BBC Radio Wales BBC Cymru Wales BBC Radio Wales BBC Radio Wales BBC Radio Wales BBC Radio Gwent BBC Radio Wales Blaenau Gwent, Caerphilly, Monmouthshire, Newport & Torfaen BBC Radio Deeside BBC Radio Clwyd Denbighshire, Flintshire & Wrexham BBC Radio Ulster BBC Radio Foyle County Derry BBC Northern Ireland BBC Radio Ulster Northern Ireland BBC Radio na Gaidhealtachd BBC Radio nan Gàidheal BBC Radio nan Eilean Scotland BBC Radio Scotland BBC Scotland BBC Radio Orkney Orkney BBC Radio Shetland Shetland BBC Essex Essex BBC Radio Cambridgeshire Cambridgeshire BBC Radio Norfolk Norfolk BBC East BBC Radio Northampton BBC Northampton BBC Radio Northampton Northamptonshire BBC Radio Suffolk Suffolk BBC Radio Bedfordshire BBC Three Counties Radio Bedfordshire, Hertfordshire & North Buckinghamshire BBC Radio Derby Derbyshire (excl. -

BBC English Regions Management Review 2013/14 Management Review 2013/14 – English Regions
BBC English Regions Management Review 2013/14 Management Review 2013/14 – English Regions If you wish to find out more about the BBC’s year – including full financial statements and performance against other public commitment – then please visit: www.bbc.co.uk/annualreport Contents 01 Introduction 02 Two minute summary Front cover 04 Service performance As part of BBC Radio Cumbria’s 40th 11 Future Strategy anniversary, the radio station linked up 11 Contacts with the BBC Philharmonic, BBC Outreach 12 Senior management team and the Cumbria Music Service to create a 13 Heads of regional and local programming Cumbria Community Orchestra and Chorus Management Review 2013/14 – English Regions Management Review 2013/14 – English Regions Controller’s introduction ‘‘ We want to do all we can to play our part in helping all forms of local journalism to flourish not only inside the BBC, but outside it too.’’ Throughout the mayhem of the winter rain, the storms and In the year ahead, our specialist network of political journalists the flood surges audiences depended on our teams for news will report on, aim to make sense of, and seek to engage people and crucial information. It was a strong example of the special in the stories which matter to local communities ahead of next responsibility we have in keeping communities in touch, but year’s General Election. We will reflect the excitement of the it was also another demonstration of the unique, and highly Commonwealth Games and other major sports events. And we prized emotional bond we have with our audiences. -

New News, Future News the Challenges for Television News After Digital Switch-Over
New News, Future News The challenges for television news after Digital Switch-over An Ofcom discussion document Publication date: 26 June 2007 Foreword The prospects for television news in a fully digital era are a central element in any consideration of the future of public service broadcasting (PSB). News is regarded by viewers as the most important of all the PSB genres, and television remains by far the most used source of news for UK citizens. The role of news and information as part of the democratic process is long established, and its status is specifically underpinned in the Communications Act 2003. This report, New News, Future News, is one of a series of Ofcom studies focussing on individual topics identified in the PSB Review of 2004/05, and further discussed in the Digital PSB report of July 2006. The others are on the provision of children’s programmes and on the prospects for a Public Service Publisher. All three studies are linked to areas of particular PSB concern for the future, and set out a framework for policy consideration ahead of the next full PSB review. Other Ofcom work of relevance includes the review of Channel 4’s funding. It has not been the role of this report to come up with solutions, and no policy recommendations are put forward. Instead, the report examines the environment in which television news currently operates, and assesses how that may change in future (after digital switch-over and, in 2014, the expiry of current Channel 3 and Channel 5 licences) . It identifies particular issues that will need to be addressed and suggests some specific questions that may need to be answered. -

ROYAL TELEVISION SOCIETY Regional Centres' Awards 2004
RTS Centre Awards 2004/5 ROYAL TELEVISION SOCIETY Regional Centres’ Awards 2004 (for progs made in 2003) West of England Craft Award: Editing Nov 2004 Rupert Troskie, Granada Bristol Craft Award: Graphic Design Julian Kirby, Granada Bristol Single Item News Coverage Points West: Concorde BBC West Regional Television Personality/Presenter/Reporter Dave Harvey, BBC Points West Network Television Personality Nick Knowles, BBC Bristol Regional Current Affairs Programme West Eye View: Fight for a Life ITV West Regional Documentary Sir Frank Whittle: The Man who Shrank the World A Big Umbrella Media & Apogee Production for BBC West Regional News Programme ITV West News Regional Independent My Peeps – Programme 2 Available Light Productions for BBC West Judge’s Award – Lifetime Contribution Rowland Jones Network Drama/Drama-Documentary Historyonics: Mary Queen of Scots BBC Bristol for BBC ONE Network Factual Britain’s Boy Soldiers Testimony Films for Channel 4 Network Feature or Documentary What’s Inside Frank Sinatra’s Coffin? Granada Bristol for Channel 4 Joint Bristol/Wales Student TV Awards Animation: Incommunicado Charles Trevan, University of the West of England Factual: Tony P Gavin Porter, International Film School Wales Non-Factual: 3 From Every 2 Tim Doubleday, Adam Linzey, Joe Wylie International Film School Wales - 1 - RTS Centre Awards 2004/5 Devon & Cornwall Network Nov 2004 Ric Stein’s Food Heroes Denham Productions for BBC TWO Non-Broadcast Champions at Home Coleridge Leisure/Entertainment Will the Real Basil Fawlty Please Stand -

Ms Claire Jones Broadcast Journalist BBC South West Broadcasting
Francis Fernandes Information Governance Borough Secretary and Monitoring Officer The Guildhall Solicitor MBA; LLM; LLB; LARTPI St Giles Square Northampton NN1 1DE Tel: (01604) 838536 Fax: (01604) 837057 Minicom: (01604) 838970 Ms Claire Jones Our Ref No: NBC3962-1056/15 Broadcast Journalist Officer: David Taylor BBC South West Broadcasting House Tel: 01604 838536 Seymour Road Email: [email protected] Plymouth PL3 5BD Date: 29th October 2015 Email: claire.jones03 at bbc.co.uk Dear Ms Jones, Information Request NBC3962-1056/15 I am writing to respond to your request for information received by email on 30/09/2015 which has been dealt with under the Freedom of Information Act 2000 (FOI). Your request has been given the unique access to information code above. Please quote this reference on any correspondence relating to this request. In response to your request for information on Public Health Funerals for 2010-2011, 2011-2012, 2012-2013, 2013-2014, 2014-2015, the Council responds to your questions as follows: 1) How many public health funerals has this local authority arranged each year? 2) How much money has this local authority spent on public health funerals each year? Financial Year Number of public health funerals paid for Total Cost (£) by the Council 2010/2011 18 18,518.11 2011/2012 36 28,684.78 2012/2013 28 9,699.61 2013/2014 29 21,367.20 2014/2015 16 25,010 Nb. Information as to whether the funerals were part or fully funded is not readily available. Recovery is ongoing and in some cases can take a number of years to finalise. -

BBC Group Annual Report and Accounts 2018/19
BBC Group Annual Report and Accounts 2018/19 BBC Group Annual Report and Accounts 2018/19 Laid before the National Assembly for Wales by the Welsh Government Return to contents © BBC Copyright 2019 The text of this document (this excludes, where present, the Royal Arms and all departmental or agency logos) may be reproduced free of charge in any format or medium provided that it is reproduced accurately and not in a misleading context. The material must be acknowledged as BBC copyright and the document title specified. Photographs are used ©BBC or used under the terms of the PACT agreement except where otherwise identified. Permission from copyright holders must be sought before any photographs are reproduced. You can download this publication from bbc.co.uk/annualreport Designed by Emperor emperor.works Prepared pursuant to the BBC Royal Charter 2016 (Article 37) Return to contents OVERVIEW Contents About the BBC 2 Inform, Educate, Entertain 4 Highlights from the year p.2 6 Award-winning content Strategic report 8 A message from the Chairman About the BBC 10 Director-General’s statement 16 Delivering our creative remit Highlights from the year and 18 – Impartial news and information award-winning content 22 – Learning for people of all ages 26 – Creative, distinctive, quality output 34 – Reflecting the UK’s diverse communities 48 – Reflecting the UK to the world 55 Audiences and external context 56 – Audience performance and market context 58 – Performance by Service 61 – Public Service Broadcasting expenditure p.8 62 – Charitable work