(2012). Light and Vision in the Deep-Sea Benthos: I
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
W7192e19.Pdf
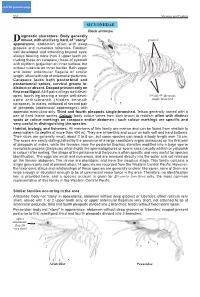
click for previous page 952 Shrimps and Prawns Sicyoniidae SICYONIIDAE Rock shrimps iagnostic characters: Body generally Drobust, with shell very hard, of “stony” grooves appearance; abdomen often with deep grooves and numerous tubercles. Rostrum well developed and extending beyond eyes, always bearing more than 3 upper teeth (in- cluding those on carapace); base of eyestalk with styliform projection on inner surface, but without tubercle on inner border. Both upper and lower antennular flagella of similar length, attached to tip of antennular peduncle. 1 Carapace lacks both postorbital and postantennal spines, cervical groove in- distinct or absent. Exopod present only on first maxilliped. All 5 pairs of legs well devel- 2 oped, fourth leg bearing a single well-devel- 3rd and 4th pleopods 4 single-branched oped arthrobranch (hidden beneath 3 carapace). In males, endopod of second pair 5 of pleopods (abdominal appendages) with appendix masculina only. Third and fourth pleopods single-branched. Telson generally armed with a pair of fixed lateral spines. Colour: body colour varies from dark brown to reddish; often with distinct spots or colour markings on carapace and/or abdomen - such colour markings are specific and very useful in distinguishing the species. Habitat, biology, and fisheries: All members of this family are marine and can be found from shallow to deep waters (to depths of more than 400 m). They are all benthic and occur on both soft and hard bottoms. Their sizes are generally small, about 2 to 8 cm, but some species can reach a body length over 15 cm. The sexes are easily distinguished by the presence of a large copulatory organ (petasma) on the first pair of pleopods of males, while the females have the posterior thoracic sternites modified into a large sperm receptacle process (thelycum) which holds the spermatophores or sperm sacs (usually whitish or yellowish in colour) after mating. -

A New Classification of the Chirostyloidea (Crustacea: Decapoda: Anomura)
Zootaxa 2687: 56–64 (2010) ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ Article ZOOTAXA Copyright © 2010 · Magnolia Press ISSN 1175-5334 (online edition) A new classification of the Chirostyloidea (Crustacea: Decapoda: Anomura) KAREEN E. SCHNABEL1 & SHANE T. AHYONG2 1National Institute of Water and Atmospheric Research, Private Bag 14901, Kilbirnie, Wellington, New Zealand. E-mail: [email protected] 2Australian Museum, 6 College Street, Sydney, NSW 2010 Australia. E-mail: [email protected] Abstract The high level classification of the Chirostyloidea Ortmann, 1892, is reviewed. Eumunididae Milne-Edwards & Bouvier, 1900, is resurrected for two genera formerly placed in the Chirostylidae Ortmann, 1892, Eumunida Smith, 1883, and Pseudomunida Haig, 1979, based on shared characteristics such as the dorsal carapace striation, presence of supraocular spines of the rostrum, dentition of the mandible, presence of an epipod and an annulated exopod flagellum of maxilliped 1. Three families are now included in the Chirostyloidea: Chirostylidae, Eumunididae and Kiwaidae. Diagnoses are provided for each family as well as a key to the families. The fossil record of the Chirostyloidea is discussed, with putative records of Eumunida in the fossil record referred to the galatheid genus Sadayoshia Baba, 1969. Key words: Galatheoidea, Chirostylidae, Eumunididae, Kiwaidae, adult somatic morphology, larval morphology, fossil record Introduction Recent focus on the phylogeny of Anomura has generated significant molecular phylogenetic information that has challenged the traditional understanding of the marine squat lobsters and porcelain crabs, the Galatheoidea, which comprised the Chirostylidae Ortmann, 1892, Galatheidae Samouelle, 1819, Porcellanidae Haworth, 1825, and Kiwaidae Macpherson, Jones & Segonzac, 2005 (e.g., Ahyong et al. -

1 Crustaceans in Cold Seep Ecosystems: Fossil Record, Geographic Distribution, Taxonomic Composition, 2 and Biology 3 4 Adiël A
1 Crustaceans in cold seep ecosystems: fossil record, geographic distribution, taxonomic composition, 2 and biology 3 4 Adiël A. Klompmaker1, Torrey Nyborg2, Jamie Brezina3 & Yusuke Ando4 5 6 1Department of Integrative Biology & Museum of Paleontology, University of California, Berkeley, 1005 7 Valley Life Sciences Building #3140, Berkeley, CA 94720, USA. Email: [email protected] 8 9 2Department of Earth and Biological Sciences, Loma Linda University, Loma Linda, CA 92354, USA. 10 Email: [email protected] 11 12 3South Dakota School of Mines and Technology, Rapid City, SD 57701, USA. Email: 13 [email protected] 14 15 4Mizunami Fossil Museum, 1-47, Yamanouchi, Akeyo-cho, Mizunami, Gifu, 509-6132, Japan. 16 Email: [email protected] 17 18 This preprint has been submitted for publication in the Topics in Geobiology volume “Ancient Methane 19 Seeps and Cognate Communities”. Specimen figures are excluded in this preprint because permissions 20 were only received for the peer-reviewed publication. 21 22 Introduction 23 24 Crustaceans are abundant inhabitants of today’s cold seep environments (Chevaldonné and Olu 1996; 25 Martin and Haney 2005; Karanovic and Brandão 2015), and could play an important role in structuring 26 seep ecosystems. Cold seeps fluids provide an additional source of energy for various sulfide- and 27 hydrocarbon-harvesting bacteria, often in symbiosis with invertebrates, attracting a variety of other 28 organisms including crustaceans (e.g., Levin 2005; Vanreusel et al. 2009; Vrijenhoek 2013). The 29 percentage of crustaceans of all macrofaunal specimens is highly variable locally in modern seeps, from 30 0–>50% (Dando et al. 1991; Levin et al. -

Texto Completo (Ver PDF)
Estado del conocimiento de los crustáceos de México María del Socorro García-Madrigal*, José Luis Villalobos-Hiriart**, Fernando Álvarez** & Rolando Bastida-Zavala* Resumen Abstract Estado del conocimiento de los crustáceos de Current knowledge of the crustaceans of México. El estudio de los crustáceos en México ha Mexico. The study of crustaceans in Mexico has tenido una historia de registros larga y discontinua. had a long and discontinuous history of records. Los primeros se realizaron principalmente por car- The first records were mainly conducted by foreign cinólogos extranjeros desde mediados del siglo XIX, carcinologists from the mid XIX century, while mientras que los investigadores mexicanos impulsa- Mexican researchers boosted the knowledge from ron el conocimiento desde el primer tercio del siglo the first third of the XX century. Mexico has topo- XX. México cuenta con condiciones topográficas y graphic and oceanographic conditions appropriate oceanográficas apropiadas para albergar una ele- to host a high diversity of niches and, therefore, vada diversidad de nichos y por lo tanto de crustá- crustaceans. Mexican crustaceans records have ceos. Los registros de crustáceos de México han sido been summarized by several Mexican authors, sintetizados por diversos autores mexicanos, por therefore, this contribution does not intend to ello, esta contribución no pretende repetir esa infor- repeat the same effort, but put into context all the mación, sino poner en contexto toda la información information generated in order to serve as a basis generada, con el objeto de que sirva como base para for resuming the systematic study of the crusta- retomar el estudio sistemático de los crustáceos de ceans from Mexico. -

How to Become a Crab: Phenotypic Constraints on a Recurring Body Plan
Preprints (www.preprints.org) | NOT PEER-REVIEWED | Posted: 25 December 2020 doi:10.20944/preprints202012.0664.v1 How to become a crab: Phenotypic constraints on a recurring body plan Joanna M. Wolfe1*, Javier Luque1,2,3, Heather D. Bracken-Grissom4 1 Museum of Comparative Zoology and Department of Organismic & Evolutionary Biology, Harvard University, 26 Oxford St, Cambridge, MA 02138, USA 2 Smithsonian Tropical Research Institute, Balboa–Ancon, 0843–03092, Panama, Panama 3 Department of Earth and Planetary Sciences, Yale University, New Haven, CT 06520-8109, USA 4 Institute of Environment and Department of Biological Sciences, Florida International University, Biscayne Bay Campus, 3000 NE 151 Street, North Miami, FL 33181, USA * E-mail: [email protected] Summary: A fundamental question in biology is whether phenotypes can be predicted by ecological or genomic rules. For over 140 years, convergent evolution of the crab-like body plan (with a wide and flattened shape, and a bent abdomen) at least five times in decapod crustaceans has been known as ‘carcinization’. The repeated loss of this body plan has been identified as ‘decarcinization’. We offer phylogenetic strategies to include poorly known groups, and direct evidence from fossils, that will resolve the pattern of crab evolution and the degree of phenotypic variation within crabs. Proposed ecological advantages of the crab body are summarized into a hypothesis of phenotypic integration suggesting correlated evolution of the carapace shape and abdomen. Our premise provides fertile ground for future studies of the genomic and developmental basis, and the predictability, of the crab-like body form. Keywords: Crustacea, Anomura, Brachyura, Carcinization, Phylogeny, Convergent evolution, Morphological integration 1 © 2020 by the author(s). -

Proceedings of the United States National Museum
descriptions of a new genus and forty-six new spp:cies of crusta(jeans of the family GALA- theid.e, with a list of the known marine SPECIES. By James E. Benedict, Assistant Curator of Marine Invertebrates. The collection of Galatheids in the United States National Museum, upon which this paper is based, began with the first dredgings of the U. S. Fish Commission steamer Albatross in 1883, and has grown as that busy ship has had opportunit}' to dredge. During the first period of its work many of the species taken were identical with those found by the U. S. Coast Survey steamer JBlake, afterwards described by A. Milne-Edwards, and in addition several new species were collected. During the voyage of the Albatross to the Pacific Ocean through the Straits of Magellan interesting addi- tions were made to the collection. Since then the greater part of the time spent by the Albatross at sea has been in Alaskan waters, where Galatheids do not seem to abound. However, occasional cruises else- where have greatly enriched the collection, notably three—one in the Gulf of California, one to the Galapagos Islands, and one to the coast of Japan and southward. The U. S. National Museum has received a number of specimens from the Museum of Natural History, Paris, and also from the Indian Museum, Calcutta. The literature of the deep-sea Galatheidie from the nature of the case is not greatly scattered. The first considerate number of species were described by A. Milne-Edwards from dredgings made by the Blake in the West Indian region. -

The Complete Mitogenome of €Lysmata Vittata
bioRxiv preprint doi: https://doi.org/10.1101/2021.08.04.455109; this version posted August 5, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. 1 The complete mitogenome of Lysmata vittata (Crustacea: 2 Decapoda: Hippolytidae) and its phylogenetic position in 3 Decapoda 4 Longqiang Zhu1,2,3, Zhihuang Zhu1,2*, Leiyu Zhu1,2, Dingquan Wang3, Jianxin 5 Wang3*, Qi Lin1,2,3* 6 1 Fisheries Research Institute of Fujian, Xiamen, China 7 2 Key Laboratory of Cultivation and High-value Utilization of Marine Organisms in Fujian Province, Xiamen, 8 China 9 3Marine Microorganism Ecological & Application Lab, Zhejiang Ocean University, Zhejiang, China 10 * [email protected] (QL); [email protected] (JW); [email protected] (ZZ) 11 12 Abstract 13 In this study, the complete mitogenome of Lysmata vittata (Crustacea: Decapoda: 14 Hippolytidae) has been determined. The genome sequence was 22003 base pairs (bp) 15 and it included thirteen protein-coding genes (PCGs), twenty-two transfer RNA genes 16 (tRNAs), two ribosomal RNA genes (rRNAs) and three putative control regions 17 (CRs). The nucleotide composition of AT was 71.50%, with a slightly negative AT 18 skewness (-0.04). Usually the standard start codon of the PCGs was ATN, while cox1, 19 nad4L and cox3 began with TTG, TTG and GTG. The canonical termination codon 20 was TAA, while nad5 and nad4 ended with incomplete stop codon T, and cox1 ended 21 with TAG. -

Download-The-Data (Accessed on 12 July 2021))
diversity Article Integrative Taxonomy of New Zealand Stenopodidea (Crustacea: Decapoda) with New Species and Records for the Region Kareen E. Schnabel 1,* , Qi Kou 2,3 and Peng Xu 4 1 Coasts and Oceans Centre, National Institute of Water & Atmospheric Research, Private Bag 14901 Kilbirnie, Wellington 6241, New Zealand 2 Institute of Oceanology, Chinese Academy of Sciences, Qingdao 266071, China; [email protected] 3 College of Marine Science, University of Chinese Academy of Sciences, Beijing 100049, China 4 Key Laboratory of Marine Ecosystem Dynamics, Second Institute of Oceanography, Ministry of Natural Resources, Hangzhou 310012, China; [email protected] * Correspondence: [email protected]; Tel.: +64-4-386-0862 Abstract: The New Zealand fauna of the crustacean infraorder Stenopodidea, the coral and sponge shrimps, is reviewed using both classical taxonomic and molecular tools. In addition to the three species so far recorded in the region, we report Spongicola goyi for the first time, and formally describe three new species of Spongicolidae. Following the morphological review and DNA sequencing of type specimens, we propose the synonymy of Spongiocaris yaldwyni with S. neocaledonensis and review a proposed broad Indo-West Pacific distribution range of Spongicoloides novaezelandiae. New records for the latter at nearly 54◦ South on the Macquarie Ridge provide the southernmost record for stenopodidean shrimp known to date. Citation: Schnabel, K.E.; Kou, Q.; Xu, Keywords: sponge shrimp; coral cleaner shrimp; taxonomy; cytochrome oxidase 1; 16S ribosomal P. Integrative Taxonomy of New RNA; association; southwest Pacific Ocean Zealand Stenopodidea (Crustacea: Decapoda) with New Species and Records for the Region. -

Occurrence of Anomuran Crabs (Crustacea: Decapoda) in Hydro Thermal Vent and Cold-Seep Communities: a Review
PROCEEDINGS OF THE BIOLOGICAL SOCIETY OF WASHINGTON l09(2):286-298. 19% Occurrence of anomuran crabs (Crustacea: Decapoda) in hydro thermal vent and cold-seep communities: a review Pierre Chevaldonne and Karine Olu (PC & KO) Laboratoire d'Ecologie Abyssale, IFREMER, B.P. 70, 29280 Plouzane, France; (PC) Present address: Institute of Marine and Coastal Sciences. Rutgers University, Dudley Road, Cook College. New Brunswick, New Jersey 08903, U.S.A. Abstract.—Crabs of the family Lithodidae are frequently encountered in the vicinity of deep-sea hydrothermal vents and cold-seeps. Together with crabs of the families Galatheidae and Chirostylidae, they are the main contributors to the scavenging/predatory fauna of these highly productive areas, and a potential vector for the export of organic carbon to the surrounding deep-sea commu nities. A review of the literature indicates that anomuran crabs have been re ported from such environments since their discovery, and occur virtually any where a reducing habitat is found. These three families are represented by at least eight genera, with at least 14 species occurring in hot vent areas, and eight in cold-seep associated communities. Two species of lithodid crabs have re Vents and seeps are environments where cently been reported from the south Bar biomass and biological production are high bados accretionary prism cold-seeps at compared to that of the surrounding abyssal depths of 1200-1700 m (Macpherson plains, due to utilization of the expelled re 1994). In addition to the description of a duced compounds by chemoautotrophic mi new species, Paralomis arethusa, Macpher croorganisms that constitute the first step of son mentioned that his new species and Li- a trophic web independent from sea-surface thodes manningi Macpherson, 1988, were production. -

Deep-Water Squat Lobsters (Crustacea: Decapoda: Anomura) from India Collected by the FORV Sagar Sampada
Bull. Natl. Mus. Nat. Sci., Ser. A, 46(4), pp. 155–182, November 20, 2020 Deep-water Squat Lobsters (Crustacea: Decapoda: Anomura) from India Collected by the FORV Sagar Sampada Vinay P. Padate1, 2, Shivam Tiwari1, 3, Sherine Sonia Cubelio1,4 and Masatsune Takeda5 1Centre for Marine Living Resources and Ecology, Ministry of Earth Sciences, Government of India. Atal Bhavan, LNG Terminus Road, Puthuvype, Kochi 682508, India 2Corresponding author: [email protected]; https://orcid.org/0000-0002-2244-8338 [email protected]; https://orcid.org/0000-0001-6194-8960 [email protected]; http://orcid.org/0000-0002-2960-7055 5Department of Zoology, National Museum of Nature and Science, Tokyo. 4–1–1 Amakubo, Tsukuba, Ibaraki 305–0005, Japan. [email protected]; https://orcid/org/0000-0002-0028-1397 (Received 13 August 2020; accepted 23 September 2020) Abstract Deep-water squat lobsters collected during five cruises of the Fishery Oceanographic Research Vessel Sagar Sampada off the Andaman and Nicobar Archipelagos (299–812 m deep) and three cruises in the southeastern Arabian Sea (610–957 m deep) are identified. They are referred to each one species of the families Chirostylidae and Sternostylidae in the Superfamily Chirostyloidea, and five species of the family Munidopsidae and three species of the family Muni- didae in the Superfamily Galatheoidea. Of altogether 10 species of 5 genera dealt herein, the Uro- ptychus species of the Chirostylidae is described as new to science, and Agononida aff. indocerta Poore and Andreakis, 2012, of the Munididae, previously reported from Western Australia and Papua New Guinea, is newly recorded from Indian waters. -

Annotated Checklist of New Zealand Decapoda (Arthropoda: Crustacea)
Tuhinga 22: 171–272 Copyright © Museum of New Zealand Te Papa Tongarewa (2011) Annotated checklist of New Zealand Decapoda (Arthropoda: Crustacea) John C. Yaldwyn† and W. Richard Webber* † Research Associate, Museum of New Zealand Te Papa Tongarewa. Deceased October 2005 * Museum of New Zealand Te Papa Tongarewa, PO Box 467, Wellington, New Zealand ([email protected]) (Manuscript completed for publication by second author) ABSTRACT: A checklist of the Recent Decapoda (shrimps, prawns, lobsters, crayfish and crabs) of the New Zealand region is given. It includes 488 named species in 90 families, with 153 (31%) of the species considered endemic. References to New Zealand records and other significant references are given for all species previously recorded from New Zealand. The location of New Zealand material is given for a number of species first recorded in the New Zealand Inventory of Biodiversity but with no further data. Information on geographical distribution, habitat range and, in some cases, depth range and colour are given for each species. KEYWORDS: Decapoda, New Zealand, checklist, annotated checklist, shrimp, prawn, lobster, crab. Contents Introduction Methods Checklist of New Zealand Decapoda Suborder DENDROBRANCHIATA Bate, 1888 ..................................... 178 Superfamily PENAEOIDEA Rafinesque, 1815.............................. 178 Family ARISTEIDAE Wood-Mason & Alcock, 1891..................... 178 Family BENTHESICYMIDAE Wood-Mason & Alcock, 1891 .......... 180 Family PENAEIDAE Rafinesque, 1815 .................................. -

An Annotated Checklist of the Marine Macroinvertebrates of Alaska David T
NOAA Professional Paper NMFS 19 An annotated checklist of the marine macroinvertebrates of Alaska David T. Drumm • Katherine P. Maslenikov Robert Van Syoc • James W. Orr • Robert R. Lauth Duane E. Stevenson • Theodore W. Pietsch November 2016 U.S. Department of Commerce NOAA Professional Penny Pritzker Secretary of Commerce National Oceanic Papers NMFS and Atmospheric Administration Kathryn D. Sullivan Scientific Editor* Administrator Richard Langton National Marine National Marine Fisheries Service Fisheries Service Northeast Fisheries Science Center Maine Field Station Eileen Sobeck 17 Godfrey Drive, Suite 1 Assistant Administrator Orono, Maine 04473 for Fisheries Associate Editor Kathryn Dennis National Marine Fisheries Service Office of Science and Technology Economics and Social Analysis Division 1845 Wasp Blvd., Bldg. 178 Honolulu, Hawaii 96818 Managing Editor Shelley Arenas National Marine Fisheries Service Scientific Publications Office 7600 Sand Point Way NE Seattle, Washington 98115 Editorial Committee Ann C. Matarese National Marine Fisheries Service James W. Orr National Marine Fisheries Service The NOAA Professional Paper NMFS (ISSN 1931-4590) series is pub- lished by the Scientific Publications Of- *Bruce Mundy (PIFSC) was Scientific Editor during the fice, National Marine Fisheries Service, scientific editing and preparation of this report. NOAA, 7600 Sand Point Way NE, Seattle, WA 98115. The Secretary of Commerce has The NOAA Professional Paper NMFS series carries peer-reviewed, lengthy original determined that the publication of research reports, taxonomic keys, species synopses, flora and fauna studies, and data- this series is necessary in the transac- intensive reports on investigations in fishery science, engineering, and economics. tion of the public business required by law of this Department.