Dodonidia Helmsii Forest Ringlet Butterfly
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A News and Events Diary from Wildlife and Conservation Groups in the Ipswich Area
Pantaloon Bee – see page 9 © Matt Garnham September - December 2018 A news and events Produced by the diary from wildlife and conservation groups in the Ipswich area BlueSnippets alien found White Admiral Lydia Woods in town Richard Stewart On the afternoon of Friday June 22nd my wife and I were walking down Westerfield Road in Ipswich and just past the gate into Christchurch Park we saw a white admiral on the pavement. It appeared to be a newly emerged While walking through Kiln Meadow on a warm adult but had probably been caught morning in July, I was more than a little surprised to in a vehicle slipstream. I cupped my hands around it, walked across the be confronted with a bright flash of blue! road and released the butterfly over the park railings. This was one of the On closer inspection I discovered a rather battered looking blue morpho butterfly new species I predicted for the park resting on the ground - not something you’d expect to see in Suffolk. These butterflies in future years as it has steadily been are generally found in Central and South America, although they are a popular choice colonising towards Ipswich. One was for butterfly houses – it’s likely this one escaped from the butterfly house situated at seen and photographed in The Dales Jimmy’s Farm. After taking some photos of this blue alien, I left the butterfly resting in a in 2015. With this in mind more patch of bindweed. While this was a lovely sight to see, hopefully it won’t be a regular honeysuckle, the larval food plant, occurrence. -
APP203875 Submissions Compilation.Pdf
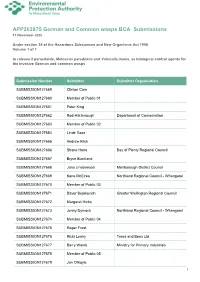
APP203875 German and Common wasps BCA Submissions 11 November 2020 Under section 34 of the Hazardous Substances and New Organisms Act 1996 Volume 1 of 1 to release 2 parasitoids, Metoecus paradoxus and Volucella inanis, as biological control agents for the invasive German and common wasps Submission Number Submitter Submitter Organisation SUBMISSION127659 Clinton Care SUBMISSION127660 Member of Public 01 SUBMISSION127661 Peter King SUBMISSION127662 Rod Hitchmough Department of Conservation SUBMISSION127663 Member of Public 02 SUBMISSION127664 Linde Rose SUBMISSION127665 Andrew Blick SUBMISSION127666 Shane Hona Bay of Plenty Regional Council SUBMISSION127667 Bryce Buckland SUBMISSION127668 Jono Underwood Marlborough District Council SUBMISSION127669 Kane McElrea Northland Regional Council - Whangarei SUBMISSION127670 Member of Public 03 SUBMISSION127671 Davor Bejakovich Greater Wellington Regional Council SUBMISSION127672 Margaret Hicks SUBMISSION127673 Jenny Dymock Northland Regional Council - Whangarei SUBMISSION127674 Member of Public 04 SUBMISSION127675 Roger Frost SUBMISSION127676 Ricki Leahy Trees and Bees Ltd SUBMISSION127677 Barry Wards Ministry for Primary Industries SUBMISSION127678 Member of Public 05 SUBMISSION127679 Jan O'Boyle 1 Submission Number Submitter Submitter Organisation Apiculture New Zealand Science and SUBMISSION127680 Sue Carter Research Focus Group SUBMISSION127681 Andrea Dorn SUBMISSION127682 Benita Wakefield Te Rūnanga o Ngāi Tahu SUBMISSION127683 Emma Edney-Browne Auckland Council SUBMISSION127684 David Hunter -

The Radiation of Satyrini Butterflies (Nymphalidae: Satyrinae): A
Zoological Journal of the Linnean Society, 2011, 161, 64–87. With 8 figures The radiation of Satyrini butterflies (Nymphalidae: Satyrinae): a challenge for phylogenetic methods CARLOS PEÑA1,2*, SÖREN NYLIN1 and NIKLAS WAHLBERG1,3 1Department of Zoology, Stockholm University, 106 91 Stockholm, Sweden 2Museo de Historia Natural, Universidad Nacional Mayor de San Marcos, Av. Arenales 1256, Apartado 14-0434, Lima-14, Peru 3Laboratory of Genetics, Department of Biology, University of Turku, 20014 Turku, Finland Received 24 February 2009; accepted for publication 1 September 2009 We have inferred the most comprehensive phylogenetic hypothesis to date of butterflies in the tribe Satyrini. In order to obtain a hypothesis of relationships, we used maximum parsimony and model-based methods with 4435 bp of DNA sequences from mitochondrial and nuclear genes for 179 taxa (130 genera and eight out-groups). We estimated dates of origin and diversification for major clades, and performed a biogeographic analysis using a dispersal–vicariance framework, in order to infer a scenario of the biogeographical history of the group. We found long-branch taxa that affected the accuracy of all three methods. Moreover, different methods produced incongruent phylogenies. We found that Satyrini appeared around 42 Mya in either the Neotropical or the Eastern Palaearctic, Oriental, and/or Indo-Australian regions, and underwent a quick radiation between 32 and 24 Mya, during which time most of its component subtribes originated. Several factors might have been important for the diversification of Satyrini: the ability to feed on grasses; early habitat shift into open, non-forest habitats; and geographic bridges, which permitted dispersal over marine barriers, enabling the geographic expansions of ancestors to new environ- ments that provided opportunities for geographic differentiation, and diversification. -

Exhibition Catalogue Natural History Illustrations by Erin Forsyth, 2018
A Few Exhibition catalogue Natural history illustrations by Erin Forsyth, 2018 TABLE OF CONTENTS ABOUT THE WORKS 5 About the artist 7 How to use this catalogue 9 TERMS AND CONDITIONS OF SALE 10 Korimako, makomako, bellbird 13 Kākāriki, Red-crowned parakeet, (Cyanoramphus novaezelandiae) 15 Moko kākāriki, Auckland green gecko (Naultinus elegans) 17 Pekapeka-tou-roa, long-tailed bat (Chalinolobus tuberculatus) 19 Pekapeka-tou-roa, long-tailed bat (Chalinolobus tuberculatus) 21 Pekapeka-tou-roa, long-tailed bat (Chalinolobus tuberculatus) 23 Ngirungiru, miromiro, South Island tomtit (Petroica macrocephala macrocephala) male 25 Kakaruwai, South Island Robin (Petroica australis) 27 Tōrea pango, variable oystercatcher (Haematopus unicolor) 29 Kererū, NZ wood pigeon (Hemiphaga novaeseelandiae) 31 Kōtare, sacred kingfisher (Todiramphus sanctus) 33 Ruru, morepork (Ninox novaeseelandiae) 35 TŪī, parsons bird (Prosthemadera novaeseelandiae) 37 Kōkako, blue-wattled crow (Callaeas wilsoni) 41 Takahe, South Island Takahe (Porphyrio hochstetteri) 43 Tūturiwhatu, NZ Dotteral (Charadrius obscurus) 45 Whio, blue duck (Hymenolaimus malacorhynchos) 47 Kahukōwhai, yellow admiral (Vanessa itea) 49 Wētāpunga, Little Barrier (Hauturu-o-Toi) giant weta (Deinacrida heteracantha) 51 Kārearea, NZ falcon (Falco novaeseelandiae) 53 Common evening brown (Melanitis leda bankia) 55 Pepe pouri, Helms' butterfly or forest ringlet (Dodonidia helmsii) 59 Kahukōwhai, yellow admiral (Vanessa itea) & Kahukura, NZ red admiral (V. gonerilla gonerilla) 63 Pepe pouri, Butler's ringlet (Erebiola butleri) & pepe pouri, black mountain ringlet (Percnodaimon merula) 67 Pīwakawaka, fantail (Rhipidura fuliginosa) 73 Weka, woodhen (Gallirallus australis) 75 Carnivorous land snail (Powelliphanta superba) 77 MYRTACEAE Studies I & II (Diptych) 79 ABOUT THE WORKS These original works are from the exhibition ‘A Few’ - the third installment in an ongoing series of natural history illustrations depicting native and resident species of Aotearoa by Erin Forsyth. -

Invertebrates – a Forgotten Group of Animals In
INVERTEBRATES – A FORGOTTEN GROUP OF ANIMALS IN INFRASTRUCTURE PLANNING? BUTTERFLIES AS TOOLS AND MODEL ORGANISMS IN SWEDEN John Askling (Phone: +46 13 12 25 75, Email: [email protected]), Calluna AB, Linköpings slot, SE-582 28 Linköping, Sweden, Fax: +46 13 12 65 95, and Karl-Olof Bergman, (Phone: +46 13 28 26 85, Email: [email protected]), Department of Biology, Linköping University, SE-581 82 Linköping, Sweden, Fax: +46 13 28 13 99 Abstract: There is a growing concern about the ecological effects of roads and railways on animals. There is increased mortality due to road kills, changes in movement patterns and changes in the physical environment in areas affected by infrastructure. A majority of all studies have been on larger mammals. There are also a growing number of studies on smaller animals like birds, amphibians and small mammals. However, the studies of invertebrates are few in comparison with vertebrates, and the knowledge of the effects of infrastructure on this group is limited. The importance of also including invertebrates in the studies of infrastructure is evident. First of all, this group of animals is the richest of species that exists. They are also ecologically important. In Sweden, a majority of the red-listed species are invertebrates. Of 4,120 red-listed species, fully 2,337 are invertebrates. Their generation times are fast, which also makes the response on changes in their environment fast, compared to mammals and birds. For that reason, invertebrates can be expected to give an indication earlier than mammals if an area is negatively affected by infrastructure. -

Phylogenetic Relatedness of Erebia Medusa and E. Epipsodea (Lepidoptera: Nymphalidae) Confirmed
Eur. J. Entomol. 110(2): 379–382, 2013 http://www.eje.cz/pdfs/110/2/379 ISSN 1210-5759 (print), 1802-8829 (online) Phylogenetic relatedness of Erebia medusa and E. epipsodea (Lepidoptera: Nymphalidae) confirmed 1 2, 3 4 MARTINA ŠEMELÁKOVÁ , PETER PRISTAŠ and ĽUBOMÍR PANIGAJ 1 Institute of Biology and Ecology, Department of Cellular Biology, Faculty of Science, Pavol Jozef Šafárik University in Košice, Moyzesova 11, 041 54 Košice, Slovakia; e-mail: [email protected] 2 Institute of Animal Physiology, Slovak Academy of Science, Soltesovej 4–6, 040 01 Košice, Slovakia 3 Department of Biology and Ecology, Faculty of Natural Sciences, Matej Bel University, Tajovskeho 40, 841 04 Banská Bystrica, Slovakia 4 Institute of Biology and Ecology, Department of Zoology, Faculty of Science, Pavol Jozef Šafárik University in Košice, Moyzesova 11, 041 54 Košice, Slovakia Key words. Lepidoptera, Nymphalidae, Erebia medusa, E. epipsodea, mtDNA, COI, ND1 Abstract. The extensive genus Erebia is divided into several groups of species according to phylogenetic relatedness. The species Erebia medusa was assigned to the medusa group and E. epipsodea to the alberganus group. A detailed study of the morphology of their copulatory organs indicated that these species are closely related and based on this E. epipsodea was transferred to the medusa group. Phylogenetic analyses of the gene sequences of mitochondrial cytochrome C oxidase subunit I (COI) and mitochondrial NADH dehydrogenase subunit 1 (ND1) confirm that E. medusa and E. epipsodea are closely related. A possible scenario is that the North American species, E. episodea, evolved after exclusion/isolation from E. medusa, whose current centre of distribution is in Europe. -

Arthropods in Linear Elements
Arthropods in linear elements Occurrence, behaviour and conservation management Thesis committee Thesis supervisor: Prof. dr. Karlè V. Sýkora Professor of Ecological Construction and Management of Infrastructure Nature Conservation and Plant Ecology Group Wageningen University Thesis co‐supervisor: Dr. ir. André P. Schaffers Scientific researcher Nature Conservation and Plant Ecology Group Wageningen University Other members: Prof. dr. Dries Bonte Ghent University, Belgium Prof. dr. Hans Van Dyck Université catholique de Louvain, Belgium Prof. dr. Paul F.M. Opdam Wageningen University Prof. dr. Menno Schilthuizen University of Groningen This research was conducted under the auspices of SENSE (School for the Socio‐Economic and Natural Sciences of the Environment) Arthropods in linear elements Occurrence, behaviour and conservation management Jinze Noordijk Thesis submitted in partial fulfilment of the requirements for the degree of doctor at Wageningen University by the authority of the Rector Magnificus Prof. dr. M.J. Kropff, in the presence of the Thesis Committee appointed by the Doctorate Board to be defended in public on Tuesday 3 November 2009 at 1.30 PM in the Aula Noordijk J (2009) Arthropods in linear elements – occurrence, behaviour and conservation management Thesis, Wageningen University, Wageningen NL with references, with summaries in English and Dutch ISBN 978‐90‐8585‐492‐0 C’est une prairie au petit jour, quelque part sur la Terre. Caché sous cette prairie s’étend un monde démesuré, grand comme une planète. Les herbes folles s’y transforment en jungles impénétrables, les cailloux deviennent montagnes et le plus modeste trou d’eau prend les dimensions d’un océan. Nuridsany C & Pérennou M 1996. -

Weelsby Woods Park Management Plan 2015
WEELSBY WOODS PARK MANAGEMENT PLAN 2015 – 2020 North East Lincolnshire Council 1 FOREWORD WEELSBY WOODS PARK FIVE YEAR MANAGEMENT & MAINTENANCE PLAN 2015-2020 Parks and open spaces can be havens for wildlife, places for quiet relaxation and reflection, venues for healthy exercise, areas for play and focal points for the community. For these reasons, parks and open spaces have an important role in providing communities with a balanced and agreeable quality of life. North East Lincolnshire is therefore fortunate to have a number of high quality and accessible parks and open spaces scattered across the area. Each with its own charm; each serving its own community. Above all, parks make a key contribution to the image and identity of our local area. Our vision for parks is that by 2022, there will be a diverse network of safe, accessible and attractive green spaces that are well managed and maintained, through community participation, to enhance the quality of life, sense of well-being, health and learning opportunities for all sections of the community. The council is committed to creating spaces that are safe, clean and well maintained. In partnership with funding bodies, the Friends Group and many other partners, North East Lincolnshire Council is pleased to be able to preserve and enhance this special place for future generations to enjoy. 1 Weelsby Woods Park Management Plan 2015 - 2020 CONTENTS Contents WEELSBY WOODS PARK ........................................................................................ 1 MANAGEMENT PLAN 2015 -

Tympanal Ears in Nymphalidae Butterflies: Morphological Diversity and Tests on the Function of Hearing
Tympanal Ears in Nymphalidae Butterflies: Morphological Diversity and Tests on the Function of Hearing by Laura E. Hall A thesis submitted to the Faculty of Graduate Studies and Postdoctoral Affairs in partial fulfillment of the requirements for the degree of Master of Science in Biology Carleton University Ottawa, Ontario, Canada © 2014 Laura E. Hall i Abstract Several Nymphalidae butterflies possess a sensory structure called the Vogel’s organ (VO) that is proposed to function in hearing. However, little is known about the VO’s structure, taxonomic distribution or function. My first research objective was to examine VO morphology and its accessory structures across taxa. Criteria were established to categorize development levels of butterfly VOs and tholi. I observed that enlarged forewing veins are associated with the VOs of several species within two subfamilies of Nymphalidae. Further, I discovered a putative light/temperature-sensitive organ associated with the VOs of several Biblidinae species. The second objective was to test the hypothesis that insect ears function to detect bird flight sounds for predator avoidance. Neurophysiological recordings collected from moth ears show a clear response to flight sounds and chirps from a live bird in the laboratory. Finally, a portable electrophysiology rig was developed to further test this hypothesis in future field studies. ii Acknowledgements First and foremost I would like to thank David Hall who spent endless hours listening to my musings and ramblings regarding butterfly ears, sharing in the joy of my discoveries, and comforting me in times of frustration. Without him, this thesis would not have been possible. I thank Dr. -

Biodiversity Distribution
Waveney Open Space Needs Assessment | July 2015 Biodiversity Distribution Biodiversity refers to all of the natural world and all living organisms within it including plants, animals, bacteria and micro organisms. www.waveney.gov.uk/planningpolicy 19 Waveney Open Space Needs Assessment | July 2015 | Biodiversity Distribution www.waveney.gov.uk/planningpolicy 20 Waveney Open Space Needs Assessment | July 2015 | Biodiversity Distribution What is biodiversity? Biodiversity refers to all of the natural world and all living organisms within it, including plants, animals, bacteria and micro organisms. The convention on biodiversity defines it as: “The variability among living organisms from all sources including terrestrial, marine and other aquatic ecosystems, and the ecological complexes of which they are part; this includes diversity within species, between species and of ecosystems” (Source: Natural England website). Introduction to the biodiversity distribution assessment Waveney District has a wide range of wildlife and habitats, including coastline, parkland, arable fields, rivers, hedges and woodlands. Many of these are extremely valuable in their own right and require protection and enhancement. These sites often also form part of a wider network of sites and wildlife corridors that increase the range of habitats that can support local wildlife. Networks of biologically valuable sites often have greater value than each of the sites individually. For this reason Waveney District Council wants to map ecological sites and networks to better understand how they can function alongside other types of green infrastructure. The Waveney District Council Biodiversity Audit was completed in 2007 by Suffolk Wildlife Trust. This biodiversity audit included officially designated sites, county wildlife sites and other sites that were considered to have ecological value. -

Tysoe(Upper Middle)
Stratford-on-Avon District Council Ecological and Geological Study of Local Service Villages Settlement Assessment: Tysoe (Upper & Middle) Designated sites: Status and Name Area in Grid Description hectares ref. LWS potential sites SP34G3 Upper Tysoe Fields and 4.33 SP3294 Semi-natural grasslands & Verge marsh WWT Tysoe Island Reserve Key Target Notes SP34m11 and SP– Mixed grasslands and broad-leaved plantation with pond SP34g17 and SP34g18 – Mixed plantations and grasslands at manor house SP34g16 and SP34g2 – The Island mixed habitats along stream SP34g16 – Raodside verge with species rich hedgerow Habitat Description The Tysoed has a low percentage overall of high distinctiveness habitats. The main areas for habitats with high distinctiveness are around the Manor House and Farm in Upper Tysoe and the area around the Tysoe Island Trust Reserve which is heavily scrubbed and wooded section along the stream with some remnants of semi-improved grassland. Distinctiveness Phase 1 habitats No. of sites Area in hectares Score High A111 Broad-leaved semi-natural woodland 4 1.79 12 A5 Orchard (commercial) 1 0.11 3 B22 Semi-improved neutral grassland 5 2.33 15 B5 Marsh/marshy grassland 1 0.35 3 G1 Standing water 3 0.04 9 G2 Running water 6 0.52 18 Sub Total 20 5.14 60 Moderate A112 Broad-leaved plantation 7 2.27 14 B6 Semi-improved neutral grassland 7 14.24 14 J112 Allotments 1 0.71 2 J113 Set-aside 2 1.24 2 Sub Total 17 18.45 32 Low A122 Coniferous plantation 1 0.56 1 A132 Mixed plantation 2 0.36 2 B4 Improved grassland 55 113.85 55 C31 Tall ruderal 1 0.08 2 J11 Arable 19 138.19 19 J12 Amenity grassland 12 7.09 24 J4 Bare ground 1 0.46 1 Sub Total 91 260.58 104 Totals 128 284.17 196 Geological Description Middle Tysoe has considerable potential for its early Jurassic geology (Charmouth Mudstone Formation), with opportunities for palaeontological discoveries. -

Coenonympha Oedippus (FABRICIUS, 1787) (Lepidoptera: Nymphalidae) in Slovenia 7 Tatjana Celik & Rudi Verovnik
Editorial: Oedippus in Oedippus 5 26 (2010) Matthias Dolek, Christian Stettmer, Markus Bräu & Josef Settele Distribution, habitat preferences and population ecology of the False Ringlet Coenonympha oedippus (FABRICIUS, 1787) (Lepidoptera: Nymphalidae) in Slovenia 7 Tatjana Celik & Rudi Verovnik False Ringlet Coenonympha oedippus (FABRICIUS, 1787) (Lepidoptera: Nymphalidae) in Croatia: current status, population dynamics and conservation management 16 Martina Šašić False Ringlet Coenonympha oedippus (FABRICIUS, 1787) (Lepidoptera: Nymphalidae) in Poland: state of knowledge and conservation prospects 20 Marcin Sielezniew, Krzysztof Pałka, Wiaczesław Michalczuk, Cezary Bystrowski, Marek Hołowiński & Marek Czerwiński Ecology of Coenonympha oedippus (FABRICIUS, 1787) (Lepidoptera: Nymphalidae) in Italy 25 Simona Bonelli, Sara Canterino & Emilio Balletto Structure and size of a threatened population of the False Ringlet Coenonympha oedippus (FABRICIUS, 1787) (Lepidoptera: Nymphalidae) in Hungary 31 Noémi Örvössy, Ágnes Vozár, Ádám Kőrösi, Péter Batáry & László Peregovits Concerning the situation of the False Ringlet Coenonympha oedippus (FABRICIUS, 1787) (Lepidoptera: Nymphalidae) in Switzerland 38 Goran Dušej, Emmanuel Wermeille, Gilles Carron & Heiner Ziegler Habitat requirements, larval development and food preferences of the German population of the False Ringlet Coenonympha oedippus (FABRICIUS, 1787) (Lepidoptera: Nymphalidae) – Research on the ecological needs to develop management tools 41 Markus Bräu, Matthias Dolek & Christian Stettmer