The Systematics and Genetics of Tomatoes on the Galápagos Islands (Solanum, Solanaceae)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Template for Two-Page Abstracts in Word 97 (PC)
GEOLOGIC MAPPING OF THE LUNAR SOUTH POLE QUADRANGLE (LQ-30). S.C. Mest1,2, D.C. Ber- man1, and N.E. Petro2, 1Planetary Science Institute, 1700 E. Ft. Lowell, Suite 106, Tucson, AZ 85719-2395 ([email protected]); 2Planetary Geodynamics Laboratory, Code 698, NASA GSFC, Greenbelt, MD 20771. Introduction: In this study we use recent image, the surface [7]. Impact craters display morphologies spectral and topographic data to map the geology of the ranging from simple to complex [7-9,24] and most lunar South Pole quadrangle (LQ-30) at 1:2.5M scale contain floor deposits distinct from surrounding mate- [1-7]. The overall objective of this research is to con- rials. Most of these deposits likely consist of impact strain the geologic evolution of LQ-30 (60°-90°S, 0°- melt; however, some deposits, especially on the floors ±180°) with specific emphasis on evaluation of a) the of the larger craters and basins (e.g., Antoniadi), ex- regional effects of impact basin formation, and b) the hibit low albedo and smooth surfaces and may contain spatial distribution of ejecta, in particular resulting mare. Higher albedo deposits tend to contain a higher from formation of the South Pole-Aitken (SPA) basin density of superposed impact craters. and other large basins. Key scientific objectives in- Antoniadi Crater. Antoniadi crater (D=150 km; clude: 1) Determining the geologic history of LQ-30 69.5°S, 172°W) is unique for several reasons. First, and examining the spatial and temporal variability of Antoniadi is the only lunar crater that contains both a geologic processes within the map area. -

Genome Skimming for Phylogenomics
Genome skimming for phylogenomics Steven Andrew Dodsworth School of Biological and Chemical Sciences, Queen Mary University of London, Mile End Road, London E1 4NS, UK. Submitted in partial fulfilment of the requirements of the degree of Doctor of Philosophy November 2015 1 Statement of originality I, Steven Andrew Dodsworth, confirm that the research included within this thesis is my own work or that where it has been carried out in collaboration with, or supported by others, that this is duly acknowledged and my contribution indicated. Previously published material is also acknowledged and a full list of publications is given in the Appendix. Details of collaboration and publications are given at the start of each chapter, as appropriate. I attest that I have exercised reasonable care to ensure that the work is original, and does not to the best of my knowledge break any UK law, infringe any third party’s copyright or other Intellectual Property Right, or contain any confidential material. I accept that the College has the right to use plagiarism detection software to check the electronic version of the thesis. I confirm that this thesis has not been previously submitted for the award of a degree by this or any other university. The copyright of this thesis rests with the author and no quotation from it or information derived from it may be published without the prior written consent of the author. Signature: Date: 16th November 2015 2 Frontispiece: Nicotiana burbidgeae Symon at Dalhousie Springs, South Australia. 2014. Photo: S. Dodsworth. 3 Acknowledgements Firstly, I would like to thank my PhD supervisors, Professor Andrew Leitch and Professor Mark Chase. -

Insects of Western North America 4. Survey of Selected Insect Taxa of Fort Sill, Comanche County, Oklahoma 2
Insects of Western North America 4. Survey of Selected Insect Taxa of Fort Sill, Comanche County, Oklahoma 2. Dragonflies (Odonata), Stoneflies (Plecoptera) and selected Moths (Lepidoptera) Contributions of the C.P. Gillette Museum of Arthropod Diversity Colorado State University Survey of Selected Insect Taxa of Fort Sill, Comanche County, Oklahoma 2. Dragonflies (Odonata), Stoneflies (Plecoptera) and selected Moths (Lepidoptera) by Boris C. Kondratieff, Paul A. Opler, Matthew C. Garhart, and Jason P. Schmidt C.P. Gillette Museum of Arthropod Diversity Department of Bioagricultural Sciences and Pest Management Colorado State University, Fort Collins, Colorado 80523 March 15, 2004 Contributions of the C.P. Gillette Museum of Arthropod Diversity Colorado State University Cover illustration (top to bottom): Widow Skimmer (Libellula luctuosa) [photo ©Robert Behrstock], Stonefly (Perlesta species) [photo © David H. Funk, White- lined Sphinx (Hyles lineata) [photo © Matthew C. Garhart] ISBN 1084-8819 This publication and others in the series may be ordered from the C.P. Gillette Museum of Arthropod Diversity, Department of Bioagricultural Sciences, Colorado State University, Fort Collins, Colorado 80523 Copyrighted 2004 Table of Contents EXECUTIVE SUMMARY……………………………………………………………………………….…1 INTRODUCTION…………………………………………..…………………………………………….…3 OBJECTIVE………………………………………………………………………………………….………5 Site Descriptions………………………………………….. METHODS AND MATERIALS…………………………………………………………………………….5 RESULTS AND DISCUSSION………………………………………………………………………..…...11 Dragonflies………………………………………………………………………………….……..11 -

ECO-Ssls for Pahs
Ecological Soil Screening Levels for Polycyclic Aromatic Hydrocarbons (PAHs) Interim Final OSWER Directive 9285.7-78 U.S. Environmental Protection Agency Office of Solid Waste and Emergency Response 1200 Pennsylvania Avenue, N.W. Washington, DC 20460 June 2007 This page intentionally left blank TABLE OF CONTENTS 1.0 INTRODUCTION .......................................................1 2.0 SUMMARY OF ECO-SSLs FOR PAHs......................................1 3.0 ECO-SSL FOR TERRESTRIAL PLANTS....................................4 5.0 ECO-SSL FOR AVIAN WILDLIFE.........................................8 6.0 ECO-SSL FOR MAMMALIAN WILDLIFE..................................8 6.1 Mammalian TRV ...................................................8 6.2 Estimation of Dose and Calculation of the Eco-SSL ........................9 7.0 REFERENCES .........................................................16 7.1 General PAH References ............................................16 7.2 References Used for Derivation of Plant and Soil Invertebrate Eco-SSLs ......17 7.3 References Rejected for Use in Derivation of Plant and Soil Invertebrate Eco-SSLs ...............................................................18 7.4 References Used in Derivation of Wildlife TRVs .........................25 7.5 References Rejected for Use in Derivation of Wildlife TRV ................28 i LIST OF TABLES Table 2.1 PAH Eco-SSLs (mg/kg dry weight in soil) ..............................4 Table 3.1 Plant Toxicity Data - PAHs ..........................................5 Table 4.1 -

DNA Variation in a Diversity Panel of Tomato Genetic Resources
J. AMER.SOC.HORT.SCI. 146(5):339–345. 2021. https://doi.org/10.21273/JASHS05066-21 DNA Variation in a Diversity Panel of Tomato Genetic Resources Joanne A. Labate Plant Genetic Resources Unit, Agricultural Research Service, U.S. Department of Agriculture, 630 W. North St., Geneva, NY 14456 ADDITIONAL INDEX WORDS. genotyping by sequencing, germplasm, Plant Variety Protection, single nucleotide polymorphism, Solanum lycopersicum, Solanum pimpinellifolium ABSTRACT. A diversity panel of 190 National Plant Germplasm System (NPGS) tomato (Solanum lycopersicum) acces- sions was genotyped using genotyping by sequencing. These originated from 31 countries and included fresh market, ornamental, processing, breeders’ lines, landraces, and home gardening types, as well as six different accessions of the economically valuable cultivar San Marzano. Most of the 34,531 discovered single nucleotide polymorphisms were rare and therefore excluded from downstream analyses. A total of 3713 high-quality, mapped single nucleotide polymorphisms that were present in at least two accessions were used to estimate genetic distances and population structure. Results showed that these phenotypically and geographically diverse NPGS tomato accessions were closely related to each other. However, a subset of divergent genotypes was identified that included landraces from primary centers of diversity (South America), secondary centers of diversity (Italy, Taiwan, and France), and genotypes that originated from wild species through 20th century breeding for disease resistance (e.g., ‘VFNT Cherry’). Extreme variant accessions produce cultivated fruit traits in a background that contains many wild or primitive genes. These accessions are promising sources of novel genes for continued crop improvement. Vegetable producers require an abundance of genetic diver- for purposes of breeding, research, and higher education. -
Relative Ages
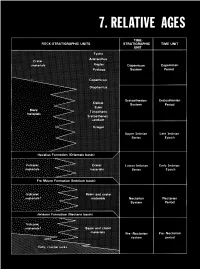
CONTENTS Page Introduction ...................................................... 123 Stratigraphic nomenclature ........................................ 123 Superpositions ................................................... 125 Mare-crater relations .......................................... 125 Crater-crater relations .......................................... 127 Basin-crater relations .......................................... 127 Mapping conventions .......................................... 127 Crater dating .................................................... 129 General principles ............................................. 129 Size-frequency relations ........................................ 129 Morphology of large craters .................................... 129 Morphology of small craters, by Newell J. Fask .................. 131 D, method .................................................... 133 Summary ........................................................ 133 table 7.1). The first three of these sequences, which are older than INTRODUCTION the visible mare materials, are also dominated internally by the The goals of both terrestrial and lunar stratigraphy are to inte- deposits of basins. The fourth (youngest) sequence consists of mare grate geologic units into a stratigraphic column applicable over the and crater materials. This chapter explains the general methods of whole planet and to calibrate this column with absolute ages. The stratigraphic analysis that are employed in the next six chapters first step in reconstructing -

DMAAC – February 1973
LUNAR TOPOGRAPHIC ORTHOPHOTOMAP (LTO) AND LUNAR ORTHOPHOTMAP (LO) SERIES (Published by DMATC) Lunar Topographic Orthophotmaps and Lunar Orthophotomaps Scale: 1:250,000 Projection: Transverse Mercator Sheet Size: 25.5”x 26.5” The Lunar Topographic Orthophotmaps and Lunar Orthophotomaps Series are the first comprehensive and continuous mapping to be accomplished from Apollo Mission 15-17 mapping photographs. This series is also the first major effort to apply recent advances in orthophotography to lunar mapping. Presently developed maps of this series were designed to support initial lunar scientific investigations primarily employing results of Apollo Mission 15-17 data. Individual maps of this series cover 4 degrees of lunar latitude and 5 degrees of lunar longitude consisting of 1/16 of the area of a 1:1,000,000 scale Lunar Astronautical Chart (LAC) (Section 4.2.1). Their apha-numeric identification (example – LTO38B1) consists of the designator LTO for topographic orthophoto editions or LO for orthophoto editions followed by the LAC number in which they fall, followed by an A, B, C or D designator defining the pertinent LAC quadrant and a 1, 2, 3, or 4 designator defining the specific sub-quadrant actually covered. The following designation (250) identifies the sheets as being at 1:250,000 scale. The LTO editions display 100-meter contours, 50-meter supplemental contours and spot elevations in a red overprint to the base, which is lithographed in black and white. LO editions are identical except that all relief information is omitted and selenographic graticule is restricted to border ticks, presenting an umencumbered view of lunar features imaged by the photographic base. -

INDEX to 1915-1919 Obituaries in the Canton Repository
INDEX To 1915-1919 Obituaries in the Canton Repository Published by the Stark County District Library of Canton, Ohio 2005 Index to 1915-1919 Obituaries in the Canton Repository The following is an alphabetical listing, by the deceased’s surname, of obituaries that appeared in the Canton, Ohio newspaper, The Repository. It was compiled by the library staff during the course of the year in the hope that it would be a useful tool to genealogy, as well as other researchers. To use this index, simply locate the name of the person whose obituary you wish to find. The entries will appear as follows: Miller William A. (Estella M.) Mrs. 1924 Jan. 13 30 The first column is the name of the deceased. The second gives the date on which the item appeared. And the third tells the page number containing the obituary. You may find one name with multiple listings showing different dates and locations in the paper. This indicates consecutive listings for that individual. I encourage you to view each for additional information. Index to 1915-1919 Obituaries in the Canton Repository Surname Given Name Maiden Title Year Mth Day Pg ? Mary 1918 Apr. 18 15 Aaron Benjamin 1918 Nov. 27 2 Abbott Lawrence C. 1919 Mar. 14 24 Abels infant 1919 July 16 6 Abherve Minnie Jacobs Mrs. 1918 Dec. 13 33 Abrams Leo 1917 Apr. 24 2 Abt Leo 1918 Apr. 27 1 Abvrezis Macone 1917 Sept. 17 2 Acker L. E. 1918 Jan. 13 26 Acker Leonard E. 1918 Jan. 12 5 Acker Sarah Mrs. -

Inflorescence Development in Two Tomato Species
111 NOTE / NOTE Inflorescence development in two tomato species N. Welty, C. Radovich, T. Meulia, and E. van der Knaap Abstract: The inflorescence of tomato has been characterized as either a cyme or raceme. Cymose inflorescences are de- terminate, whereas racemose inflorescences are indeterminate. In this study, we addressed the discrepancy in inflorescence architecture by analyzing the morphology of a wild relative of tomato Solanum pimpinellifolium L. and four domesticated Solanum lycopersicum L. lines. Careful observation of developing inflorescences of both species showed a bifurcation of the meristem into a determinate floral and an indeterminate inflorescence meristem. Interestingly, higher fruit carpel num- ber was associated with delayed floral development, which might give the impression of determinate growth in some of the lines. Nevertheless, our results demonstrated that tomato inflorescences are indeterminate in nature regardless of the line studied. Floral buds were formed concomitantly with the development of the inflorescence meristem and not on the flanks of the peduncle, a characteristic of racemose growth. Thus, tomato inflorescences should be classified as a cyme with the note that the inflorescence meristem does not terminate into a flower and, in fact, maintains indeterminacy. In ad- dition, S. pimpinellifolium produced many more flowers in a highly regular manner when compared with the cultivated types. This demonstrated the usefulness of wild relatives of tomato as a tool to further understand flower and fruit devel- opment in this crop species. Key words: inflorescence, tomato, cyme, raceme, meristem, bifurcation. Re´sume´ : On a caracte´rise´ l’inflorescence de la tomate comme une cyme ou un race`me. -

Discoveries of Mass Independent Isotope Effects in the Solar System: Past, Present and Future Mark H
Reviews in Mineralogy & Geochemistry Vol. 86 pp. 35–95, 2021 2 Copyright © Mineralogical Society of America Discoveries of Mass Independent Isotope Effects in the Solar System: Past, Present and Future Mark H. Thiemens Department of Chemistry and Biochemistry University of California San Diego La Jolla, California 92093 USA [email protected] Mang Lin State Key Laboratory of Isotope Geochemistry Guangzhou Institute of Geochemistry, Chinese Academy of Sciences Guangzhou, Guangdong 510640 China University of Chinese Academy of Sciences Beijing 100049 China [email protected] THE BEGINNING OF ISOTOPES Discovery and chemical physics The history of the discovery of stable isotopes and later, their influence of chemical and physical phenomena originates in the 19th century with discovery of radioactivity by Becquerel in 1896 (Becquerel 1896a–g). The discovery catalyzed a range of studies in physics to develop an understanding of the nucleus and the properties influencing its stability and instability that give rise to various decay modes and associated energies. Rutherford and Soddy (1903) later suggested that radioactive change from different types of decay are linked to chemical change. Soddy later found that this is a general phenomenon and radioactive decay of different energies and types are linked to the same element. Soddy (1913) in his paper on intra-atomic charge pinpointed the observations as requiring the observations of the simultaneous character of chemical change from the same position in the periodic chart with radiative emissions required it to be of the same element (same proton number) but differing atomic weight. This is only energetically accommodated by a change in neutrons and it was this paper that the name “isotope” emerges. -

Flora Mediterranea 26
FLORA MEDITERRANEA 26 Published under the auspices of OPTIMA by the Herbarium Mediterraneum Panormitanum Palermo – 2016 FLORA MEDITERRANEA Edited on behalf of the International Foundation pro Herbario Mediterraneo by Francesco M. Raimondo, Werner Greuter & Gianniantonio Domina Editorial board G. Domina (Palermo), F. Garbari (Pisa), W. Greuter (Berlin), S. L. Jury (Reading), G. Kamari (Patras), P. Mazzola (Palermo), S. Pignatti (Roma), F. M. Raimondo (Palermo), C. Salmeri (Palermo), B. Valdés (Sevilla), G. Venturella (Palermo). Advisory Committee P. V. Arrigoni (Firenze) P. Küpfer (Neuchatel) H. M. Burdet (Genève) J. Mathez (Montpellier) A. Carapezza (Palermo) G. Moggi (Firenze) C. D. K. Cook (Zurich) E. Nardi (Firenze) R. Courtecuisse (Lille) P. L. Nimis (Trieste) V. Demoulin (Liège) D. Phitos (Patras) F. Ehrendorfer (Wien) L. Poldini (Trieste) M. Erben (Munchen) R. M. Ros Espín (Murcia) G. Giaccone (Catania) A. Strid (Copenhagen) V. H. Heywood (Reading) B. Zimmer (Berlin) Editorial Office Editorial assistance: A. M. Mannino Editorial secretariat: V. Spadaro & P. Campisi Layout & Tecnical editing: E. Di Gristina & F. La Sorte Design: V. Magro & L. C. Raimondo Redazione di "Flora Mediterranea" Herbarium Mediterraneum Panormitanum, Università di Palermo Via Lincoln, 2 I-90133 Palermo, Italy [email protected] Printed by Luxograph s.r.l., Piazza Bartolomeo da Messina, 2/E - Palermo Registration at Tribunale di Palermo, no. 27 of 12 July 1991 ISSN: 1120-4052 printed, 2240-4538 online DOI: 10.7320/FlMedit26.001 Copyright © by International Foundation pro Herbario Mediterraneo, Palermo Contents V. Hugonnot & L. Chavoutier: A modern record of one of the rarest European mosses, Ptychomitrium incurvum (Ptychomitriaceae), in Eastern Pyrenees, France . 5 P. Chène, M. -

John Hooper - Pioneer British Batman
NEWSLETTER AND PROCEEDINGS OF THE LINNEAN SOCIETY OF LONDON VOLUME 26 x NUMBER xJULY 2010 THE LINNEAN SOCIETY OF LONDON Registered Charity Number 220509 Burlington House, Piccadilly, London W1J 0BF Tel. (+44) (0)20 7434 4479; Fax: (+44) (0)20 7287 9364 e-mail: [email protected]; internet: www.linnean.org President Secretaries Council Dr Vaughan Southgate BOTANICAL The Officers and Dr Sandra D Knapp Prof Geoffrey Boxshall Vice-Presidents Prof Mark Chase Dr Mike Fay ZOOLOGICAL Prof Dianne Edwards Dr Sandra D Knapp Dr Malcolm Scoble Mr Alistair Land Dr Keith Maybury Dr Terry Langford Dr Malcolm Scoble EDITORIAL Mr Brian Livingstone Dr John R Edmondson Prof Geoff Moore Treasurer Ms Sara Oldfield Professor Gren Ll Lucas OBE COLLECTIONS Dr Sylvia Phillips Mrs Susan Gove Mr Terence Preston Executive Secretary Dr Mark Watson Dr Ruth Temple Librarian Dr David Williams Mrs Lynda Brooks Prof Patricia Willmer Financial Controller/Membership Mr Priya Nithianandan Deputy Librarian Conservator Mr Ben Sherwood Ms Janet Ashdown Building and Office Manager Ms Victoria Smith Honorary Archivist Conservation Assistant Ms Gina Douglas Ms Lucy Gosnay Communications Manager Ms Claire Inman Special Publications and Education Manager Ms Leonie Berwick Office Assistant Mr Tom Helps THE LINNEAN Newsletter and Proceedings of the Linnean Society of London ISSN 0950-1096 Edited by Brian G Gardiner Editorial ................................................................................................................ 1 Society News..............................................................................................................