Solubility Behaviour of Antimony(Iii) and Antimony(V) Solids in Basic Aqueous Solutions to 300°C
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Critical Mineral - Antimony
Critical Mineral - Antimony Listed as one of the 35 critical minerals by the U.S. Government, antimony strengthens metal in munitions, is used in batteries, solar panels, and wind turbines, and therefore plays an important role in our defense and energy industries. Today, China and Russia control the world’s supply of antimony, leaving the U.S. without a direct source of this mineral which is important to our national, economic, and environmental future. Perpetua Resources is developing the only commercially viable antimony mine in the U.S. to source supply for new battery and solar technologies, as well as alloys critical for national security products. CRITICAL TO THE GREEN ECONOMY Critical minerals are vital to national and economic security, as well as the future green economy. Domestic sourcing and production of all critical minerals has bi-partisan political support. Antimony is critical in the use of bearings for wind turbines, glass clarifcation for solar energy projects, and cable sheathing for wiring and as an important component in many electrical and solid state circuitry components. For defense, antimony use is critical in fame retardants, primers, and ammunition. Antimony has current and projected widespread use for the U.S. green energy sectors. Recent studies point to antimony playing a substantial role in the development of large-scale, safe, affordable battery storage technology. A limited supply of antimony and lack of advanced new antimony development projects are considered key risks to this technology moving forward. SUPPLY Currently, 92% antimony Based on the 2020 Feasibility Study the Stibnite Gold production is dominated by Project is expected to produce enough antimony to supply approximately 30% of U.S. -

Generation of Carbon Dioxide and Mobilization of Antimony Trioxide by Fungal Decomposition of Building Materials John D
University of South Florida Scholar Commons Graduate Theses and Dissertations Graduate School 3-25-2005 Generation of Carbon Dioxide and Mobilization of Antimony Trioxide by Fungal Decomposition of Building Materials John D. Krause University of South Florida Follow this and additional works at: https://scholarcommons.usf.edu/etd Part of the American Studies Commons Scholar Commons Citation Krause, John D., "Generation of Carbon Dioxide and Mobilization of Antimony Trioxide by Fungal Decomposition of Building Materials" (2005). Graduate Theses and Dissertations. https://scholarcommons.usf.edu/etd/730 This Dissertation is brought to you for free and open access by the Graduate School at Scholar Commons. It has been accepted for inclusion in Graduate Theses and Dissertations by an authorized administrator of Scholar Commons. For more information, please contact [email protected]. Generation of Carbon Dioxide and Mobilization of Antimony Trioxide by Fungal Decomposition of Building Materials by John D. Krause A dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy Department of Environmental and Occupational Health College of Public Health University of South Florida Major Professor: Yehia Y. Hammad, Sc.D. Noreen D. Poor, Ph.D. Ann C. Debaldo, Ph.D. Diane Te Strake, Ph.D. Date of Approval: March 25, 2005 Keywords: mold, mould, carbon dioxide, antimony trioxide, flame retardant © Copyright 2005, John D. Krause Dedication For their love, support, patience and understanding throughout this endeavor, I dedicate this work to my family, daughter, and most of all, my loving wife. Acknowledgements I would like to acknowledge the following individuals and companies for their assistance in this research. -

Gsaüiiveiwibte
'C or GSAÜIIVEIWIBTE CUflIIITIIIi MïtiTHI AJIAlfïïf ,7 -' y/ . •'• .'7. 's -i, . \ STELLINGEN BEHORENDE BIJ HEJ FROEFSCHRIffT VAN R. FURLER 1. De episoomtheorie over het ontstaan van het mitochon- drion is weinig plausibel. R.A, Ratt and H.R. Mahler, Science 221 O972),575 2, Doordat S. Cirendini et al. de dragergassnelheid aan 'aet einde van een chromatografische kolom gebruiken, ontstaat een geflatteerd beeld van de weergegeven re- sultaten. Tevens is het niet mogelijk een dragergas- snelheid te berekenen zonder dat men de interstitiële porositeit kent» S, Cirendini, J. Vermont, J.C. Gressin and CL. Guilleain , J. Chromat. 84 (1973),24 3. De in de mode zijnde bepaling van RNA-moleculair ge- wichten door metingen aan formaldehyde behandelde RNA's berust op dubieuze aannamen. J.M. Kaper and M.E. v/aterworth,Virology 51 (1973),183 T.O. Diener and D.R. Smith, Virology *£ (^973), 359 M.M. El Manna and G. Bruening, Virology 56 (1973),198 4, Op grond van de zeer grote verschillen in stralingska- rakteristiek van de isotopen 1-131 en 1-123 is het streven van isotopenproducenten om een zo 'schoon' mogelijk 1-123 voor diagnostische doeleinden te leve- ren in strijd met de volksgezondheid, doordat de ver- tragingen#die dit oplevert onnodige stralingsbelasting voor patiënten veroorzaakt. H. ïlishiyama et al. J.Nucl.Med. 1£ (1974),261 5« De analogie die Gilbert et al. opmerken tussen de "exchange peak" in de kolom vloaistofchromatografie met behulp van ionenwisselaar en de luchtpi.ek bij gaschromatografie is twijfelachtig. T.W. Gilbert and R.A, Dobbs, Analyt.Chem. 45 (1>73), 1390. -

Circular of the Bureau of Standards No. 539 Volume 10: Standard X-Ray
:ationa.u d H.W. BIS <T be Libra.ry, Reisrence book not to 1965 JVPR 1 6 from ibe lib s ary. taken NBS C | RCULAR 539 VOLUME 10 Standard X-ray Diffraction Powder Patterns UNITED STATES DEPARTMENT OF COMMERCE NATIONAL BUREAU OF STANDARDS THE NATIONAL BUREAU OF STANDARDS Functions and Activities The Functions of the National Bureau of Standards are set forth in the Act of Congress, March 3, 1901, as amended by Congress in Public Law 619, 1950. These include the development and maintenance of the national standards of measurement and the provision of means and methods for making measurements consistent with these standards; the determination of physical constants and properties of materials; the development of methods and instruments for testing materials, devices, and structures; advisory services to government agencies on scientific and technical problems; in- vention and development of devices to serve special needs of the Government; and the development of standard practices, codes, and specifications. The work includes basic and applied research, development, engineering, instrumentation, testing, evaluation, calibration services, and various consultation and information services. Research projects are also performed for other government agencies when the work relates to and supplements the basic program of the Bureau or when the Bureau’s unique competence is required. The scope of activities is suggested by the listing of divisions and sections on the inside of the back cover. Publications The results of the Bureau’s work take the form of either actual equipment and devices or pub- lished papers. These papers appear either in the Bureau’s own series of publications or in the journals of professional and scientific societies. -

4. Inorganic Flame Retardants. Plastics Can Be Given Flame Retardant Characteristics by Introducing Elements of Organic, Inorganic and Halogen Origin
4. Inorganic Flame Retardants. Plastics can be given flame retardant characteristics by introducing elements of organic, inorganic and halogen origin. Such elements include magnesium, aluminium, phosphorous, molybdenum, antimony, tin, chlorine and bromine. Flame retardants are added in either the manufacturing step of the polymer or the compounding step of the polymeric article. Phosphorous bromine and chlorine are usually included as some organic compound. Inorganic flame retardants are usually added together with other flame retardants to provide a more efficient flame retardant action through synergism. Halogen flame retardants usually need an addition of about 40% in order to be effective, and this affects the properties of the polymer quite negatively. Structural integrity of the polymer article is often very important, and a drastic decrease in strength and other mechanical properties is simply not acceptable. The efficiency of halogen flame retardants is often enhanced by the addition of inorganic flame retardants. A smaller mass percentage halogen flame retardant is now needed, so the adverse effect on the polymer properties is also reduced (Touval, 1993) . 4.1 Antimony Compounds The antimony compounds used for flame retardancy include antimony trioxide, antimony pentoxide and antimony-metal compounds. In 1990 in the United States alone, the use of antimony trioxide amounted to 20 000 metric tons just for the flame retardancy of plastics. Antimony oxide is readily found in nature but in very impure form. This is not suitable for 29 direct use as flame retardant, so antimony oxide is often rather produced from antimony metal. There are therefore many different grades of antimony oxide that can be used for flame retardants. -

WO 2015/121485 Al 20 August 2015 (20.08.2015) P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2015/121485 Al 20 August 2015 (20.08.2015) P O P C T (51) International Patent Classification: (81) Designated States (unless otherwise indicated, for every B01J 23/18 (2006.01) B01J 35/02 (2006.01) kind of national protection available): AE, AG, AL, AM, B01J 23/22 (2006.01) C07C 15/24 (2006.01) AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, B01J 27/198 (2006.01) B01J 37/00 (2006.01) BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, B01J 35/00 (2006.01) DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, HN, HR, HU, ID, IL, IN, IR, IS, JP, KE, KG, KN, KP, KR, (21) International Application Number: KZ, LA, LC, LK, LR, LS, LU, LY, MA, MD, ME, MG, PCT/EP2015/053270 MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, OM, (22) International Filing Date: PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, SC, 17 February 2015 (17.02.2015) SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. (25) Filing Language: English (84) Designated States (unless otherwise indicated, for every (26) Publication Language: English kind of regional protection available): ARIPO (BW, GH, (30) Priority Data: GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, ST, SZ, 14155332. -

Properties and Human Exposure; Sanford Garner; Roc; Jan. 24, 2018
Draft RoC Monograph on Antimony Trioxide Properties and Human Exposure Sanford Garner, PhD Integrated Laboratory Systems, Inc. Contractor supporting the Office of the Report on Carcinogens National Institute of Environmental Health Sciences January 24, 2018 Properties Antimony and antimony compounds • Antimony is a metalloid found in nature in over 100 mineral species – Exists as four oxidation states: -3, 0, +3 and +5 • +3 (trivalent) and +5 (pentavalent) are most common in environmental, biological, and geochemical systems – Antimony species can undergo transformation during manufacturing processes, in the environment, or in vivo • Elemental antimony is a silver-white metal used to make alloys • Antimony(III) trioxide exists as an odorless white powder or polymorphic crystals Properties Solubility of antimony oxides and antimony metal is higher in biological fluids than in water • Antimony trioxide: 3.3 mg/L in water • Antimony pentoxide: 0.043 mg/L in water • Antimony metal: Insoluble in water Source: ECHA Registration Dossiers for diantimony pentoxide and diantimony trioxide. Properties and Human Exposure Human Exposure Human Exposure A significant number of people in the United States are exposed to antimony(III) trioxide based on: • Consumption (~ 70 million lb/yr; 1 producer and 10 importers reported in the United States) in manufacturing • Widespread use in industrial applications (e.g., 273 companies in the flame retardant industry) • Occupational exposure • General population exposure – Consumer products – Environmental exposure Uses of Antimony(III) Trioxide Antimony(III) trioxide is the most commercially significant form of processed antimony • Workers in formulation, processing, and manufacturing of consumer products are exposed to antimony(III) trioxide Consumer Formulation Processing products flame retardant e.g., furniture, flame retardant plastics (including electrical and synergist PVC), textiles, electronic equipment rubbers e.g., PET containers PET packaging and PET catalyst for water, soft drinks, fibers etc. -

FS Heavy Metals and Metalloids Final
DETOX Program Fact Sheet – Heavy Metals and Metalloids DETOX Program Hazardous Substances Fact Sheet Heavy Metals and Metalloids 1 DETOX Program Fact Sheet – Heavy Metals and Metalloids Content 1 Background .................................................................................................................... 3 2 Definition ........................................................................................................................ 3 3 Legal Aspects ................................................................................................................. 4 4 Hazardous Properties and Exposure .............................................................................. 4 4.1 Hazardous Properties .............................................................................................. 4 4.2 Exposure ................................................................................................................. 5 5 Sources for Heavy Metals and Metalloids in production of textiles .................................. 6 6 Alternative and Substitute Substances ........................................................................... 7 2 DETOX Program Fact Sheet – Heavy Metals and Metalloids 1 Background Heavy metals and metalloids are constituents of specific dyes and pigments, tanning chemicals for leather, catalysts in fiber production, printing pastes, as part of flame retardants and many more. They can also be found in natural fibers due to absorption by plants through soil or from fertilizers. Metals -

CLARC Excerpt
Washington State Department of Ecology - CLARC Air Table (Methods B and C) - February 2021 February 2021 S S CPFi S CPFo S Air Air RfC o RfDi o Inhalation RfDo o Oral o Air Air Method C Method C Inhalation u Inhalation IUR u Cancer Oral u Cancer u Method B Method B Noncancer Cancer Reference Reference Inhalation Potency Reference Potency Noncancer Cancer (Eq. 750-1 (Eq. 750-2 Chemical Data Links to r r r r Concentration c Dose Unit Risk c Factor Dose c Factor c (Eq. 750-1) (Eq. 750-2) adjusted) adjusted) 3 3 -1 CAS No. Group Chemical Name Important Notes (mg/m ) e (mg/kg-day) (µg/m ) e (kg-day/mg) (mg/kg-day) e (kg-day/mg) e (µg/m³) (µg/m³) (µg/m³) (µg/m³) 83-32-9 PAHs acenaphthene 6.00E-02 I 30560-19-1 Pesticides acephate 1.20E-03 O 75-07-0 VOCs acetaldehyde 9.00E-03 I 2.57E-03 2.20E-06 I 7.70E-03 4.10E+00 1.10E+00 9.00E+00 1.10E+01 34256-82-1 Pesticides acetochlor 2.00E-02 I 67-64-1 VOCs acetone 3.10E+01 A 8.86E+00 9.00E-01 I 1.40E+04 3.10E+04 75-86-5 VOCs acetone cyanohydrin 2.00E-03 X 5.71E-04 9.10E-01 2.00E+00 75-05-8 VOCs acetonitrile 6.00E-02 I 1.71E-02 2.70E+01 6.00E+01 98-86-2 SVOCs acetophenone 1.00E-01 I 62476-59-9 Herbicides acifluorfen, sodium 1.30E-02 I 107-02-8 VOCs acrolein 2.00E-05 I 5.71E-06 5.00E-04 I 9.10E-03 2.00E-02 79-06-1 VOCs acrylamide 6.00E-03 I 1.71E-03 1.00E-04 I-M 3.50E-01 2.00E-03 I 5.00E-01 I-M 2.70E+00 6.60E-03 6.00E+00 2.50E-01 79-10-7 VOCs acrylic acid 1.00E-03 I 2.86E-04 5.00E-01 I 4.60E-01 1.00E+00 107-13-1 VOCs acrylonitrile 2.00E-03 I 5.71E-04 6.80E-05 I 2.38E-01 4.00E-02 A 5.40E-01 I 9.10E-01 3.70E-02 -

123. Antimony
1998:11 The Nordic Expert Group for Criteria Documentation of Health Risks from Chemicals 123. Antimony John Erik Berg Knut Skyberg Nordic Council of Ministers arbete och hälsa vetenskaplig skriftserie ISBN 91–7045–471–x ISSN 0346–7821 http://www.niwl.se/ah/ah.htm National Institute for Working Life National Institute for Working Life The National Institute for Working Life is Sweden's center for research and development on labour market, working life and work environment. Diffusion of infor- mation, training and teaching, local development and international collaboration are other important issues for the Institute. The R&D competence will be found in the following areas: Labour market and labour legislation, work organization and production technology, psychosocial working conditions, occupational medicine, allergy, effects on the nervous system, ergonomics, work environment technology and musculoskeletal disorders, chemical hazards and toxicology. A total of about 470 people work at the Institute, around 370 with research and development. The Institute’s staff includes 32 professors and in total 122 persons with a postdoctoral degree. The National Institute for Working Life has a large international collaboration in R&D, including a number of projects within the EC Framework Programme for Research and Technology Development. ARBETE OCH HÄLSA Redaktör: Anders Kjellberg Redaktionskommitté: Anders Colmsjö och Ewa Wigaeus Hjelm © Arbetslivsinstitutet & författarna 1998 Arbetslivsinstitutet, 171 84 Solna, Sverige ISBN 91–7045–471–X ISSN 0346-7821 Tryckt hos CM Gruppen Preface The Nordic Council is an intergovernmental collaborative body for the five countries, Denmark, Finland, Iceland, Norway and Sweden. One of the committees, the Nordic Senior Executive Committee for Occupational Environmental Matters, initiated a project in order to produce criteria documents to be used by the regulatory authorities in the Nordic countries as a scientific basis for the setting of national occupational exposure limits. -
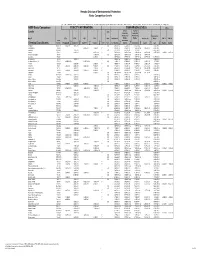
Basic Comparison Levels
Nevada Division of Environmental Protection Basic Comparison Levels Key: I=IRIS; P= PPRTV; N=NCEA; H=HEAST; A=ATSDR; O=Other Documents; CA=CalEPA S=Surrogate X=Appendix PPRTV E=Based on TEF scheme r=Route Extra Key: C = Cancer endpoint; N = Noncancer endpoint; sat = Saturation Limit; max = Ceiling Limit NDEP Basic Comparison TOXICITY INFORMATION COMPARISON LEVELS LBCLs Indoor Outdoor Levels Skin Industrial/ Industrial/ Commercial Commercial Residential May-17 SFo RfDo IUR RfCi Abs. Residential Worker Worker Ambient Air Water DAF 1 DAF 20 CAS w/o Dermal Chemical Constituents Number 1/(mg/kg-d) (mg/kg-d) (ug/m3)-1 (mg/m3) VOCc Soils Soil (mg/kg) (mg/kg) Soil (mg/kg) (µg/m3) (µg/l) (mg/kg) (mg/kg) Key Key key Key Key Key Key Key Key Acephate 30560-19-1 8.70E-03 I 4.00E-03 I 0.10 5.59E+01 C 7.52E+02 C 2.95E+02 C 7.73E+00 C Acetaldehyde 75-07-0 2.20E-06 I 9.00E-03 I V 1.23E+01 C 5.35E+01 C 1.00E+05 max 1.28E+00 C 2.55E+00 C Acetochlor 34256-82-1 2.00E-02 I 0.10 1.23E+03 N 4.67E+04 N 1.83E+04 N 6.67E+02 N Acetone 67-64-1 9.00E-01 I 3.10E+01 A V 7.04E+04 N 1.00E+05 max 1.00E+05 max 3.23E+04 N 2.05E+04 N 8.00E-01 1.60E+01 Acetone Cyanohydrin 75-86-5 2.00E-03 X 0.10 1.00E+05 max 1.00E+05 max 1.00E+05 max 2.09E+00 N Acetonitrile 75-05-8 6.00E-02 I V 1.00E+05 max 3.75E+03 N 1.00E+05 max 6.26E+01 N 1.25E+02 N Acetophenone 98-86-2 1.00E-01 I V 2.52E+03 sat 2.52E+03 sat 2.52E+03 sat 3.34E+03 N Acetylaminofluorene, 2- 53-96-3 3.80E+00 CA 1.30E-03 CA 0.10 1.28E-01 C 1.72E+00 C 6.75E-01 C 2.16E-03 C 1.77E-02 C Acrolein 107-02-8 5.00E-04 I 2.00E-05 I V -

Toxicological Profile for Antimony
ANTIMONY AND COMPOUNDS 11 CHAPTER 2. HEALTH EFFECTS 2.1 INTRODUCTION The primary purpose of this chapter is to provide public health officials, physicians, toxicologists, and other interested individuals and groups with an overall perspective on the toxicology of antimony. It contains descriptions and evaluations of toxicological studies and epidemiological investigations and provides conclusions, where possible, on the relevance of toxicity and toxicokinetic data to public health. When available, mechanisms of action are discussed along with the health effects data; toxicokinetic mechanistic data are discussed in Section 3.1. A glossary and list of acronyms, abbreviations, and symbols can be found at the end of this profile. To help public health professionals and others address the needs of persons living or working near hazardous waste sites, the information in this section is organized by health effect. These data are discussed in terms of route of exposure (inhalation, oral, and dermal) and three exposure periods: acute (≤14 days), intermediate (15–364 days), and chronic (≥365 days). As discussed in Appendix B, a literature search was conducted to identify relevant studies examining health effect endpoints. Figure 2-1 provides an overview of the database of studies in humans or experimental animals included in this chapter of the profile. These studies evaluate the potential health effects associated with inhalation, oral, or dermal exposure to antimony, but may not be inclusive of the entire body of literature. A systematic review of the scientific evidence of the health effects associated with exposure to antimony was also conducted; the results of this review are presented in Appendix C.