(12) Patent Application Publication (10) Pub. No.: US 2007/0021303 A1 Rosinger Et Al
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Glufosinate-Tolerant Cotton: Tolerance and Weed
GLUFOSINATE-TOLERANT COTTON: TOLERANCE AND WEED MANAGEMENT by LESLI KRISTEN BLAIR, B.S. A THESIS IN CROP SCIENCE Submitted to the Graduate Faculty of Texas Tech University in Partial Fulfillment of the Requirements for the Degree of MASTER OF SCIENCE Approved Accepted Deamof the Graduate School December, 1991 ^^•ft:;^ ACKNOWLEDGEMENTS 1i would like to extend my heartfelt gratitude to the members that served on 7 '^ '^* my advisory committee for their time, effort, and assistance. I would like to thank Dr. Dotray for his guidance and direction in the preparation of this thesis; Dr. Keeling for his instruction and support in monitoring these experiments; Dr. Gannaway for his knowledge and assistance in the biotechnology and breeding associated with this project; and Dr. Thompson for her friendship and support in making career decisions. Without all of their guidance, I could never have achieved the goals that we set. I wish to thank all of my fellow graduate students in Weed Science for their help in completing this project. I would especially like to thank Alan Helm for his endless help collecting all of the first year data, Ginger Light for her help in clarifying concepts, and LeAnna Lyon for her help with the last year's project. Their support, friendship, and help on this project have been immeasurable. I would like to express my sincerest thanks to AgrEvo and TxCot for their funding of this research. My thanks also go to the people at the USDA-ARS in Lubbock for all of their help before and after I became involved with this research. -

WO 2013/037955 Al 21 March 2013 (21.03.2013) P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization I International Bureau (10) International Publication Number (43) International Publication Date WO 2013/037955 Al 21 March 2013 (21.03.2013) P O P C T (51) International Patent Classification: (81) Designated States (unless otherwise indicated, for every A01N 25/00 (2006.01) A OIN 43/653 (2006.01) kind of national protection available): AE, AG, AL, AM, A0 41/06 (2006.01) A01N 37/50 (2006.01) AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, (21) Number: International Application DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, PCT/EP2012/068096 HN, HR, HU, ID, IL, IN, IS, JP, KE, KG, KM, KN, KP, (22) International Filing Date: KR, KZ, LA, LC, LK, LR, LS, LT, LU, LY, MA, MD, 14 September 2012 (14.09.2012) ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, OM, PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, (25) Filing Language: English RW, SC, SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, (26) Publication Language: English TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. (30) Priority Data: 1118 1702.9 16 September 201 1 (16.09.201 1) EP (84) Designated States (unless otherwise indicated, for every kind of regional protection available): ARIPO (BW, GH, (71) Applicant (for all designated States except US): BAYER GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, SZ, TZ, INTELLECTUAL PROPERTY GMBH [DE/DE]; Al- UG, ZM, ZW), Eurasian (AM, AZ, BY, KG, KZ, RU, TJ, fred-Nobel-Str. -

WO 2013/020985 Al 14 February 2013 (14.02.2013) P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization I International Bureau (10) International Publication Number (43) International Publication Date WO 2013/020985 Al 14 February 2013 (14.02.2013) P O P C T (51) International Patent Classification: (74) Agent: BAYER INTELLECTUAL PROPERTY A 47/06 (2006.01) A01P 7/02 (2006.01) GMBH; Alfred-Nobel-Str. 10, 40789 Monheim (DE). A01N 57/20 (2006.01) A01P 7/04 (2006.01) (81) Designated States (unless otherwise indicated, for every A01P 5/00 (2006.01) kind of national protection available): AE, AG, AL, AM, (21) International Application Number: AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, PCT/EP2012/065469 BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, (22) International Filing Date: HN, HR, HU, ID, IL, IN, IS, JP, KE, KG, KM, KN, KP, 7 August 2012 (07.08.2012) KR, KZ, LA, LC, LK, LR, LS, LT, LU, LY, MA, MD, (25) Filing Language: English ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, OM, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, (26) Publication Language: English SC, SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, (30) Priority Data: TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, 61/521,827 10 August 201 1 (10.08.201 1) US ZW. (71) Applicant (for all designated States except US): BAYER (84) Designated States (unless otherwise indicated, for every INTELLECTUAL PROPERTY GMBH [DE/DE]; Al- kind of regional protection available): ARIPO (BW, GH, fred-Nobel-Str. -

Universal Chloroplast Integration and Expression Vectors, Transformed
(19) TZZ ¥_¥¥_T (11) EP 2 319 933 B1 (12) EUROPEAN PATENT SPECIFICATION (45) Date of publication and mention (51) Int Cl.: of the grant of the patent: C12N 15/82 (2006.01) A01H 5/00 (2006.01) 15.10.2014 Bulletin 2014/42 (21) Application number: 10181990.2 (22) Date of filing: 05.08.1998 (54) Universal chloroplast integration and expression vectors, transformed plants and products thereof Universeller Integrations- und Expressionsvektor für Chloroplasten; transformierte Pflanzen und daraus abkömmliche Produkte Vecteurs universels d’intégration et d’expression de chloroplastes, plantes transformées et produits obtenus (84) Designated Contracting States: • SVAB Z ET AL: "HIGH-FREQUENCY PLASTID AT BE CH CY DE DK ES FI FR GB GR IE IT LI LU TRANSFORMATION IN TOBACCO BY MC NL PT SE SELECTION FOR ACHIMERIC AADA GENE", PROCEEDINGS OF THE NATIONAL ACADEMY (30) Priority: 07.08.1997 US 55314 P OF SCIENCES OF THE UNITED STATES (PNAS), 23.03.1998 US 79042 P NATIONAL ACADEMY OF SCIENCE, US, vol. 90, 15.05.1998 US 79640 no. 3, 1 February 1993 (1993-02-01), pages 913-917, XP002942726, ISSN: 0027-8424, DOI: (43) Date of publication of application: DOI:10.1073/PNAS.90.3.913 11.05.2011 Bulletin 2011/19 • GADANI F ET AL: "Tobacco: a tool for plant genetic engineering research and molecular (62) Document number(s) of the earlier application(s) in farming", AGRO FOOD INDUSTRY HI-TECH, accordance with Art. 76 EPC: TEKNOSZIENZE, MILAN, IT, vol. 6, 1 March 1995 05011140.0 / 1 571 220 (1995-03-01), pages 3-6, XP002134757, 98935230.7 / 1 002 115 • STAUB, J.M. -

Literature Review of Controlling Aquatic Invasive Vegetation With
Eurasian watermilfoil in Christmas Lake, 2011 Literature Review on Controlling Aquatic Invasive Vegetation with Aquatic Herbicides Compared to Other Control Methods: Effectiveness, Impacts, and Costs Prepared for: Prepared by: Minnehaha Creek Watershed District Steve McComas Blue Water Science St. Paul, MN 55116 September 2011 1 Literature Review on Controlling Aquatic Invasive Vegetation with Aquatic Herbicides Compared to Other Control Methods: Effectiveness, Impacts, and Costs Steve McComas, Blue Water Science Table of Contents page number Introduction .................................................................................................................................................................. 1 Use of Herbicides as an Aquatic Plant Control Technique ...................................................................................... 2 How Herbicides Work and Their Mode of Action ....................................................................................................... 3 Aquatic Herbicide Impacts on Humans and the Ecosystem ....................................................................................... 8 Where to Find Sources of Specific Information on herbicide Products and Their Active Ingredients ....................... 16 Harvesting, Drawdown, and Biocontrol as Aquatic Plant Control Techniques ................................................... 17 Summary of Control Techniques for Non-Native Curlyleaf Pondweed and Eurasian Watermilfoil ................... 25 Control Techniques for Other -

Pesticide and Veterinary Drug Standard Item List
Pesticide and Veterinary Drug Standard Item List 2021.7.1 edition The grade listed in this list are subject to change without notice. * Please check the https://labchem-wako.fujifilm.com/us/index.html for the latest information. TraceSure® and Traceable Reference Material (TRM) have a purity guarantee by an * external organization in addition to our grade. Component Compound CASNo. Code No. Product Name Glade Volume Molecular Formula MW Purity Abamectin 71751-41-2 016-20361 Abamectin Standard for Pesticide Residue Analysis 200mg 95.0+% (HPLC)(アベルメクチンB1a+アベルメクチンB1b) Abamectin 8,9-Z-Avermectin B1a 113665-89-7 018-24961 8,9-Z-Avermectin B1a Standard for Pesticide Residue Analysis 10mg C48H72O14 873.08 95.0+% (HPLC) Acephate Acephate 30560-19-1 015-08954 Acephate Standard for Pesticide Residue Analysis 100mg C4H10NO3PS 183.17 98.0+% (cGC)※ Acequinocyl 57960-19-7 018-18591 Acequinocyl Standard for Pesticide Residue Analysis 200mg C24H32O4 384.51 98.5+% (HPLC), 97.0+% (qNMR) Acequinocyl Acequinocyl-hydroxy 57960-31-3 011-18601 Acequinocyl-hydroxy Standard for Pesticide Residue Analysis 200mg C22H30O3 342.47 99.0+% (HPLC), 99.0+% (qNMR) Acetaminophen Acetaminophen 103-90-2 015-22651 Acetaminophen Standard for HPLC 100mg CH3CONHC6H4OH 151.16 98.0+% (HPLC), 97.0+% (qNMR) Acetamiprid 160430-64-8 010-16493 Acetamiprid Standard for Pesticide Residue Analysis 100mg C10H11ClN4 222.67 98.0+% (cGC) ※ Acetamiprid Acetamiprid Metabolite IM-2-1 - 019-25851 Acetamiprid Metabolite IM-2-1 Standard for Pesticide Residue Analysis 100mg C9H9ClN4 208.65 98.0+% -

By USDA APHIS BRS Document Control Officer at 2:18 Pm, Dec 11
CBI Deleted EXECUTIVE SUMMARY The Animal and Plant Health Inspection Service (APHIS) of the United States (U.S.) Department of Agriculture (USDA) has responsibility under the Plant Protection Act (Title IV Pub. L. 106-224, 114 Stat. 438, 7 U.S.C. § 7701-7772) to prevent the introduction and dissemination of plant pests into the U.S. APHIS regulation 7 CFR § 340.6 provides that an applicant may petition APHIS to evaluate submitted data to determine that a particular regulated article does not present a plant pest risk and no longer should be regulated. If APHIS determines that the regulated article does not present a plant pest risk, the petition is granted, thereby allowing unrestricted introduction of the article. Monsanto Company is submitting this request to APHIS for a determination of nonregulated status for the new biotechnology-derived maize product, MON 87429, any progeny derived from crosses between MON 87429 and conventional maize, and any progeny derived from crosses of MON 87429 with biotechnology-derived maize that have previously been granted nonregulated status under 7 CFR Part 340. Product Description Monsanto Company has developed herbicide tolerant MON 87429 maize, which is tolerant to the herbicides dicamba, glufosinate, aryloxyphenoxypropionate (AOPP) acetyl coenzyme A carboxylase (ACCase) inhibitors (so called “FOPs” herbicides such as quizalofop) and 2,4-dichlorophenoxyacetic acid (2,4-D). In addition, it provides tissue-specific glyphosate tolerance to facilitate the production of hybrid maize seeds. MON 87429 contains -

Weed Science: Principles and Practices, 4Th Edition
WEED SCIENCE Principles and Practices OURTH EDITION Thomas J. Monaco North Carolina State University Raleigh, North Carolina Stephen C. Weller Purdue University West Lafayette, Indiana loyd M. Ashton University of California Davis, California WEED SCIENCE Principles and Practices WEED SCIENCE Principles and Practices OURTH EDITION Thomas J. Monaco North Carolina State University Raleigh, North Carolina Stephen C. Weller Purdue University West Lafayette, Indiana loyd M. Ashton University of California Davis, California This book is printed on acid-free paper. Copyright © 2002 by John Wiley & Sons, Inc., New York. All rights reserved. Published simultaneously in Canada. No part of this publication may be reproduced, stored in a retrieval system or transmitted in any form or by any means, electronic, mechanical, photocopying, recording, scanning or otherwise, except as permitted under Sections 107 or 108 or the 1976 United States Copyright Act, without either the prior written permission of the Publisher, or authorization through payment of the appropriate per-copy fee to the Copyright Clearance Center, 222 Rosewood Drive, Danvers, MA 01923, (978) 750-8400, fax (978) 750-4744. Requests to the Publisher for permission should be addressed to the Permissions Department, John Wiley & Sons, Inc., 605 Third Avenue, New York, NY 10158-0012, (212) 850-6011, fax (212) 850-6008, E-Mail: [email protected]. This publication is designed to provide accurate and authoritative information in regard to the subject matter covered. It is sold with the understanding that the publisher is not engaged in rendering professional services. If professional advice or other expert assistance is required, the services of a competent professional person should be sought. -

<Title of Work, Centered
Weed Management in Bollgard II® XtendFlexTM Cotton by Justin L. Spradley, B.S. A Thesis In Crop Science Submitted to the Graduate Faculty of Texas Tech University in Partial Fulfillment of the Requirements for the Degree of MASTER OF SCIENCE Approved Dr. J. Wayne Keeling Co-Chair of Committee Dr. Peter A. Dotray Co-chair of Committee Dr. Jason E. Woodward Dr. Guy H. Loneragan Dr. Mark Sheridan Dean of the Graduate School May, 2014 Copyright 2014, Justin L. Spradley Texas Tech University, Justin L. Spradley, May 2014 ACKNOWLEDGMENTS I first thank Jesus for His divine love, creation, and being my savior. I would like to thank my wife Cryctal for all the sacrifices she has made in order for me to accomplish my work, and my daughter Saige for her sacrifice of time with her daddy. I would like to thank Drs. Wayne Keeling and Peter Dotray for allowing me the opportunity to work with their programs. I also thank them for the many hours spent on my trials and thesis work. I have learned a tremendous amount from these gentlemen and appreciate greatly who they are. I would also like to thank Dr. Jason Woodward for all the time he has spent with me in and out of class and at work as well. I gained knowledge of plant pathology from this man, the number one plant pathologist. I thank Dr. Guy Loneragan for being part of my committee and a friend at Texas Tech. I thank you for your time reviewing my work as well as being the professional animal scientist you are. -
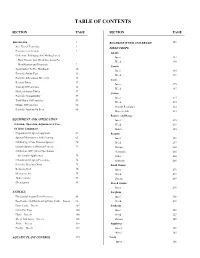
Table of Contents
TABLE OF CONTENTS SECTION PAGE SECTION PAGE Introduction 1 ROADSIDE WEED AND BRUSH 139 Safe Use Of Pesticides 1 FIELD CROPS Pesticide Certification 2 Alfalfa Collection, Packaging And Mailing Insect, Insect 153 Plant Disease And Weed Specimens For Weed 159 Identification and Diagnosis 7 Canola Contributors To The Handbook 10 Insect 165 Pesticide Safety Tips 12 Weed 171 Pesticide Information Directory 15 Corn Reentry Times 17 Insect 175 Toxicity Of Pesticides 18 Weed 187 Mode of Action Tables 47 Cotton Pesticide Compatibility 59 Insect 215 Tank Mixes Of Pesticides 59 Weed 225 Misuse Of Pesticides 60 Growth Regulators 232 Pesticide Toxicity To Bees 60 Harvest Aids 233 Pasture And Range EQUIPMENT AND APPLICATION Insect 235 Selection, Operation, Adjustment & Care Weed 239 Of Spray Equipment Brush 245 Preparation Of Spray Equipment 62 Peanuts Sprayer Maintenance And Cleaning 65 Insect 251 Calibrating A Low Pressure Sprayer 70 Weed 257 Ground Sprayer Calibration Process 72 Disease 263 Calibration Of Fertilizer Distributors Nematode 265 & Granular Applicators 74 Foliar 266 Calculation Of Spray Percentage 74 Soilborne 269 Pesticide Dilution Charts 75 Small Grains Reducing Drift 76 Insect 271 Measurements 79 Weed 277 Abbreviations 85 Disease 289 Chemigation 85 Stored Grains Insect 299 ANIMALS Sorghum Fly Control Around Farm Premises 89 Insect 303 Beef Cattle And Nonlactating Dairy Cattle – Insects 94 Weed 311 Dairy Cattle – Insects 102 Soybeans Cattle Ear Tags 105 Insect 321 Horse – Insects 106 Weed 327 Sheep And Goats – Insects 108 Disease 349 -

Method for Improved Utilization of the Production Potential of Transgenic Plants Introduction
(19) TZZ ZZ_T (11) EP 2 204 094 A1 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: (51) Int Cl.: 07.07.2010 Bulletin 2010/27 A01N 43/653 (2006.01) A01N 47/02 (2006.01) C12N 15/82 (2006.01) (21) Application number: 08173031.9 (22) Date of filing: 29.12.2008 (84) Designated Contracting States: (71) Applicant: Bayer CropScience AG AT BE BG CH CY CZ DE DK EE ES FI FR GB GR 40789 Monheim (DE) HR HU IE IS IT LI LT LU LV MC MT NL NO PL PT RO SE SI SK TR (72) Inventor: The designation of the inventor has not Designated Extension States: yet been filed AL BA MK RS (54) Method for improved utilization of the production potential of transgenic plants Introduction (57) The Invention relates to methods for increasing with an effective amount of an insecticidal composition the production potential of plants and/or controlling pests comprising a in plants with at least one transgenic modification related component A, selected from the group consisting of im- to yield increase as compared to a corresponding wild- idacloprid, thiacloprid, clothianidin, acetamiprid, dinote- type plant, comprising treating the location where the furan, nitenpyram, and thiamethoxam; and a plant with at least one transgenic modification is growing component B, selceted from the group consisting of or is expected to grow and/or the transgenic plant with fipronil and ethiprole. at least one transgenic modification or propagation ma- terial of the plant with at least one transgenic modification EP 2 204 094 A1 Printed by Jouve, 75001 PARIS (FR) EP 2 204 094 A1 Description Introduction 5 [0001] The invention relates to a method for improving the utilization of the production potential of transgenic plants. -
Application No. AU FFICE 2012201147 B2
(12) STANDARD PATENT (11) Application No. AU 2012201147 B2 (19) AUSTRALIAN PATENT 0 FFICE (54) Title Insecticidal mixture containing gamma-cyhalothrin (51) International Patent Classification(s) A01N 25/30 (2006.01) A01N 47/34(2006.01) A01N 37/28 (2006.01) A01N 47/40 (2006.01) A01N 41/02 (2006.01) A01N 47/42 (2006.01) A01N 43/36 (2006.01) A01N 51/00 (2006.01) A01N 43/40 (2006.01) A01N 53/00 (2006.01) A01N 43/56 (2006.01) A01N 53/08 (2006.01) A01N 43/58 (2006.01) A01N 57/14 (2006.01) A01N 43/653 (2006.01) A01N 57/76 (2006.01) A01N 47/18 (2006.01) A01N 63/02 (2006.01) A01N 47/20 (2006.01) (21) Application No: 2012201147 (22) Date of Filing: 2012.02.27 (43) Publication Date: 2012.03.22 (43) Publication Journal Date: 2012.03.22 (44) Accepted Journal Date: 2013.09.12 (62) Divisional of: 2011201771 (71) Applicant(s) Syngenta Limited (72) Inventor(s) Clough, Martin Stephen (74) Agent / Attorney Phillips Ormonde Fitzpatrick, 367 Collins Street, Melbourne, VIC, 3000 (56) Related Art US 4362722 A ABSTRACT 2012 A mixture of gamma-cyhalothrin and chlorpyrifos. An insecticidal composition comprising gamma- Feb cyhalothrin, chlorpyrifos and an insecticidally inert carrier or diluents. A method of combatting and 5 controlling insect, acarine or nematode pests at a locus which comprises treating the pests or the locus 27 of the pests with an effective amount of a mixture comprising gamma-cyhalothrin and chlorpyrifos. 2012201147 «filename* T ♦' .. AUSTRALIA Patents Act 2012 COMPLETE SPECIFICATION (ORIGINAL) Feb Class Int.