Tinea Capitis Favus-Like Appearance: Problem of Diagnosis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Estimated Burden of Serious Fungal Infections in Ghana
Journal of Fungi Article Estimated Burden of Serious Fungal Infections in Ghana Bright K. Ocansey 1, George A. Pesewu 2,*, Francis S. Codjoe 2, Samuel Osei-Djarbeng 3, Patrick K. Feglo 4 and David W. Denning 5 1 Laboratory Unit, New Hope Specialist Hospital, Aflao 00233, Ghana; [email protected] 2 Department of Medical Laboratory Sciences, School of Biomedical and Allied Health Sciences, College of Health Sciences, University of Ghana, P.O. Box KB-143, Korle-Bu, Accra 00233, Ghana; [email protected] 3 Department of Pharmaceutical Sciences, Faculty of Health Sciences, Kumasi Technical University, P.O. Box 854, Kumasi 00233, Ghana; [email protected] 4 Department of Clinical Microbiology, School of Medical Sciences, Kwame Nkrumah University of Science and Technology, Kumasi 00233, Ghana; [email protected] 5 National Aspergillosis Centre, Wythenshawe Hospital and the University of Manchester, Manchester M23 9LT, UK; [email protected] * Correspondence: [email protected] or [email protected] or [email protected]; Tel.: +233-277-301-300; Fax: +233-240-190-737 Received: 5 March 2019; Accepted: 14 April 2019; Published: 11 May 2019 Abstract: Fungal infections are increasingly becoming common and yet often neglected in developing countries. Information on the burden of these infections is important for improved patient outcomes. The burden of serious fungal infections in Ghana is unknown. We aimed to estimate this burden. Using local, regional, or global data and estimates of population and at-risk groups, deterministic modelling was employed to estimate national incidence or prevalence. Our study revealed that about 4% of Ghanaians suffer from serious fungal infections yearly, with over 35,000 affected by life-threatening invasive fungal infections. -

Introduction
Notes Introduction 1. In the book, we use the terms ‘fungal infections’ and ‘mycoses’ (or singular fungal infection and mycosis) interchangeably, mostly following the usage of our historical actors in time and place. 2. The term ‘ringworm’ is very old and came from the circular patches of peeled, inflamed skin that characterised the infection. In medicine at least, no one understood it to be associated with worms of any description. 3. Porter, R., ‘The patient’s view: Doing medical history from below’, Theory and Society, 1985, 14: 175–198; Condrau, F., ‘The patient’s view meets the clinical gaze’, Social History of Medicine, 2007, 20: 525–540. 4. Burnham, J. C., ‘American medicine’s golden age: What happened to it?’, Sci- ence, 1982, 215: 1474–1479; Brandt, A. M. and Gardner, M., ‘The golden age of medicine’, in Cooter, R. and Pickstone, J. V., eds, Medicine in the Twentieth Century, Amsterdam, Harwood, 2000, 21–38. 5. The Oxford English Dictionary states that the first use of ‘side-effect’ was in 1939, in H. N. G. Wright and M. L. Montag’s textbook: Materia Med Pharma- col & Therapeutics, 112, when it referred to ‘The effects which are not desired in any particular case are referred to as “side effects” or “side actions” and, in some instances, these may be so powerful as to limit seriously the therapeutic usefulness of the drug.’ 6. Illich, I., Medical Nemesis: The Expropriation of Health, London, Calder & Boyars, 1975. 7. Beck, U., Risk Society: Towards a New Society, New Delhi, Sage, 1992, 56 and 63; Greene, J., Prescribing by Numbers: Drugs and the Definition of Dis- ease, Baltimore, Johns Hopkins University Press, 2007; Timmermann, C., ‘To treat or not to treat: Drug research and the changing nature of essential hypertension’, Schlich, T. -

Fungal Infections from Human and Animal Contact
Journal of Patient-Centered Research and Reviews Volume 4 Issue 2 Article 4 4-25-2017 Fungal Infections From Human and Animal Contact Dennis J. Baumgardner Follow this and additional works at: https://aurora.org/jpcrr Part of the Bacterial Infections and Mycoses Commons, Infectious Disease Commons, and the Skin and Connective Tissue Diseases Commons Recommended Citation Baumgardner DJ. Fungal infections from human and animal contact. J Patient Cent Res Rev. 2017;4:78-89. doi: 10.17294/2330-0698.1418 Published quarterly by Midwest-based health system Advocate Aurora Health and indexed in PubMed Central, the Journal of Patient-Centered Research and Reviews (JPCRR) is an open access, peer-reviewed medical journal focused on disseminating scholarly works devoted to improving patient-centered care practices, health outcomes, and the patient experience. REVIEW Fungal Infections From Human and Animal Contact Dennis J. Baumgardner, MD Aurora University of Wisconsin Medical Group, Aurora Health Care, Milwaukee, WI; Department of Family Medicine and Community Health, University of Wisconsin School of Medicine and Public Health, Madison, WI; Center for Urban Population Health, Milwaukee, WI Abstract Fungal infections in humans resulting from human or animal contact are relatively uncommon, but they include a significant proportion of dermatophyte infections. Some of the most commonly encountered diseases of the integument are dermatomycoses. Human or animal contact may be the source of all types of tinea infections, occasional candidal infections, and some other types of superficial or deep fungal infections. This narrative review focuses on the epidemiology, clinical features, diagnosis and treatment of anthropophilic dermatophyte infections primarily found in North America. -

Serious Fungal Infections in Peru
Eur J Clin Microbiol Infect Dis DOI 10.1007/s10096-017-2924-9 ORIGINAL ARTICLE Serious fungal infections in Peru B. Bustamante1 & D. W. Denning2 & P. E. Campos3 Received: 21 December 2016 /Accepted: 21 December 2016 # Springer-Verlag Berlin Heidelberg 2017 Abstract Epidemiological data about mycotic diseases are This first attempt to assess the fungal burden in Peru needs limited in Peru and estimation of the fungal burden has not to be refined. We believe the figure obtained is an underesti- been previously attempted. Data were obtained from the mation, because of under diagnosis, non-mandatory reporting Peruvian National Institute of Statistics and Informatics, and lack of a surveillance system and of good data about the UNAIDS and from the Ministry of Health’s publications. size of populations at risk. We also searched the bibliography for Peruvian data on my- cotic diseases, asthma, COPD, cancer and transplants. Incidence or prevalence for each fungal disease were estimat- Introduction ed in specific populations at risk. The Peruvian population for 2015 was 31,151,543. In 2014, the estimated number of HIV/ While candidemia and invasive aspergillosis (IA) are clearly AIDS and pulmonary tuberculosis cases was 88,625, 38,581 recognized as important causes of morbidity and mortality, of them not on ART, and 22,027, respectively. A total of other mycotic agents or different clinical presentations are im- 581,174 cases of fungal diseases were estimated, representing portant in specific regions. Socio-economic and geo- approximately 1.9% of the Peruvian population. This figure ecological characteristics and size of susceptible populations includes 498,606, 17,361 and 4,431 vulvovaginal, oral and are the main determinants of variations on incidence of fungal esophageal candidiasis, respectively, 1,557 candidemia cases, infections across the world. -

Estimated Burden of Fungal Infections in Oman
Journal of Fungi Article Estimated Burden of Fungal Infections in Oman Abdullah M. S. Al-Hatmi 1,2,3,* , Mohammed A. Al-Shuhoumi 4 and David W. Denning 5 1 Department of microbiology, Natural & Medical Sciences Research Center, University of Nizwa, Nizwa 616, Oman 2 Department of microbiology, Centre of Expertise in Mycology Radboudumc/CWZ, 6500 Nijmegen, The Netherlands 3 Foundation of Atlas of Clinical Fungi, 1214GP Hilversum, The Netherlands 4 Ibri Hospital, Ministry of Health, Ibri 115, Oman; [email protected] 5 Manchester Fungal Infection Group, Manchester Academic Health Science Centre, The University of Manchester, Manchester M13 9PL, UK; [email protected] * Correspondence: [email protected]; Tel.: +968-25446328; Fax: +968-25446612 Abstract: For many years, fungi have emerged as significant and frequent opportunistic pathogens and nosocomial infections in many different populations at risk. Fungal infections include disease that varies from superficial to disseminated infections which are often fatal. No fungal disease is reportable in Oman. Many cases are admitted with underlying pathology, and fungal infection is often not documented. The burden of fungal infections in Oman is still unknown. Using disease frequencies from heterogeneous and robust data sources, we provide an estimation of the incidence and prevalence of Oman’s fungal diseases. An estimated 79,520 people in Oman are affected by a serious fungal infection each year, 1.7% of the population, not including fungal skin infections, chronic fungal rhinosinusitis or otitis externa. These figures are dominated by vaginal candidiasis, followed by allergic respiratory disease (fungal asthma). An estimated 244 patients develop invasive aspergillosis and at least 230 candidemia annually (5.4 and 5.0 per 100,000). -

Standard Methods for Fungal Brood Disease Research Métodos Estándar Para La Investigación De Enfermedades Fúngicas De La Cr
Journal of Apicultural Research 52(1): (2013) © IBRA 2013 DOI 10.3896/IBRA.1.52.1.13 REVIEW ARTICLE Standard methods for fungal brood disease research Annette Bruun Jensen1*, Kathrine Aronstein2, José Manuel Flores3, Svjetlana Vojvodic4, María 5 6 Alejandra Palacio and Marla Spivak 1Department of Plant and Environmental Sciences, University of Copenhagen, Thorvaldsensvej 40, 1817 Frederiksberg C, Denmark. 2Honey Bee Research Unit, USDA-ARS, 2413 E. Hwy. 83, Weslaco, TX 78596, USA. 3Department of Zoology, University of Córdoba, Campus Universitario de Rabanales (Ed. C-1), 14071, Córdoba, Spain. 4Center for Insect Science, University of Arizona, 1041 E. Lowell Street, PO Box 210106, Tucson, AZ 85721-0106, USA. 5Unidad Integrada INTA – Facultad de Ciencias Ags, Universidad Nacional de Mar del Plata, CC 276,7600 Balcarce, Argentina. 6Department of Entomology, University of Minnesota, St. Paul, Minnesota 55108, USA. Received 1 May 2012, accepted subject to revision 17 July 2012, accepted for publication 12 September 2012. *Corresponding author: Email: [email protected] Summary Chalkbrood and stonebrood are two fungal diseases associated with honey bee brood. Chalkbrood, caused by Ascosphaera apis, is a common and widespread disease that can result in severe reduction of emerging worker bees and thus overall colony productivity. Stonebrood is caused by Aspergillus spp. that are rarely observed, so the impact on colony health is not very well understood. A major concern with the presence of Aspergillus in honey bees is the production of airborne conidia, which can lead to allergic bronchopulmonary aspergillosis, pulmonary aspergilloma, or even invasive aspergillosis in lung tissues upon inhalation by humans. In the current chapter we describe the honey bee disease symptoms of these fungal pathogens. -

Oral Antifungals Month/Year of Review: July 2015 Date of Last
© Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35 Salem, Oregon 97301-1079 Phone 503-947-5220 | Fax 503-947-1119 Class Update with New Drug Evaluation: Oral Antifungals Month/Year of Review: July 2015 Date of Last Review: March 2013 New Drug: isavuconazole (a.k.a. isavunconazonium sulfate) Brand Name (Manufacturer): Cresemba™ (Astellas Pharma US, Inc.) Current Status of PDL Class: See Appendix 1. Dossier Received: Yes1 Research Questions: Is there any new evidence of effectiveness or safety for oral antifungals since the last review that would change current PDL or prior authorization recommendations? Is there evidence of superior clinical cure rates or morbidity rates for invasive aspergillosis and invasive mucormycosis for isavuconazole over currently available oral antifungals? Is there evidence of superior safety or tolerability of isavuconazole over currently available oral antifungals? • Is there evidence of superior effectiveness or safety of isavuconazole for invasive aspergillosis and invasive mucormycosis in specific subpopulations? Conclusions: There is low level evidence that griseofulvin has lower mycological cure rates and higher relapse rates than terbinafine and itraconazole for adult 1 onychomycosis.2 There is high level evidence that terbinafine has more complete cure rates than itraconazole (55% vs. 26%) for adult onychomycosis caused by dermatophyte with similar discontinuation rates for both drugs.2 There is low -

Tinea Capitis 2014 L.C
BJD GUIDELINES British Journal of Dermatology British Association of Dermatologists’ guidelines for the management of tinea capitis 2014 L.C. Fuller,1 R.C. Barton,2 M.F. Mohd Mustapa,3 L.E. Proudfoot,4 S.P. Punjabi5 and E.M. Higgins6 1Department of Dermatology, Chelsea & Westminster Hospital, Fulham Road, London SW10 9NH, U.K. 2Department of Microbiology, Leeds General Infirmary, Leeds LS1 3EX, U.K. 3British Association of Dermatologists, Willan House, 4 Fitzroy Square, London W1T 5HQ, U.K. 4St John’s Institute of Dermatology, Guy’s and St Thomas’ NHS Foundation Trust, St Thomas’ Hospital, Westminster Bridge Road, London SE1 7EH, U.K. 5Department of Dermatology, Hammersmith Hospital, 150 Du Cane Road, London W12 0HS, U.K. 6Department of Dermatology, King’s College Hospital, Denmark Hill, London SE5 9RS, U.K. 1.0 Purpose and scope Correspondence Claire Fuller. The overall objective of this guideline is to provide up-to- E-mail: [email protected] date, evidence-based recommendations for the management of tinea capitis. This document aims to update and expand Accepted for publication on the previous guidelines by (i) offering an appraisal of 8 June 2014 all relevant literature since January 1999, focusing on any key developments; (ii) addressing important, practical clini- Funding sources cal questions relating to the primary guideline objective, i.e. None. accurate diagnosis and identification of cases; suitable treat- ment to minimize duration of disease, discomfort and scar- Conflicts of interest ring; and limiting spread among other members of the L.C.F. has received sponsorship to attend conferences from Almirall, Janssen and LEO Pharma (nonspecific); has acted as a consultant for Alliance Pharma (nonspe- community; (iii) providing guideline recommendations and, cific); and has legal representation for L’Oreal U.K. -

Therapies for Common Cutaneous Fungal Infections
MedicineToday 2014; 15(6): 35-47 PEER REVIEWED FEATURE 2 CPD POINTS Therapies for common cutaneous fungal infections KENG-EE THAI MB BS(Hons), BMedSci(Hons), FACD Key points A practical approach to the diagnosis and treatment of common fungal • Fungal infection should infections of the skin and hair is provided. Topical antifungal therapies always be in the differential are effective and usually used as first-line therapy, with oral antifungals diagnosis of any scaly rash. being saved for recalcitrant infections. Treatment should be for several • Topical antifungal agents are typically adequate treatment weeks at least. for simple tinea. • Oral antifungal therapy may inea and yeast infections are among the dermatophytoses (tinea) and yeast infections be required for extensive most common diagnoses found in general and their differential diagnoses and treatments disease, fungal folliculitis and practice and dermatology. Although are then discussed (Table). tinea involving the face, hair- antifungal therapies are effective in these bearing areas, palms and T infections, an accurate diagnosis is required to ANTIFUNGAL THERAPIES soles. avoid misuse of these or other topical agents. Topical antifungal preparations are the most • Tinea should be suspected if Furthermore, subsequent active prevention is commonly prescribed agents for dermatomy- there is unilateral hand just as important as the initial treatment of the coses, with systemic agents being used for dermatitis and rash on both fungal infection. complex, widespread tinea or when topical agents feet – ‘one hand and two feet’ This article provides a practical approach fail for tinea or yeast infections. The pharmacol- involvement. to antifungal therapy for common fungal infec- ogy of the systemic agents is discussed first here. -
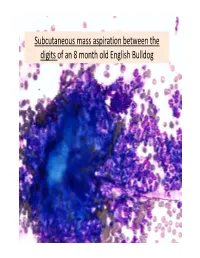
Subcutaneous Mass Aspiration Between the Digits of an 8 Month Old English Bulldog Marked Inflammation: Neutrophils & Macrophages
Subcutaneous mass aspiration between the digits of an 8 month old English Bulldog Marked inflammation: neutrophils & macrophages Hair fragment Macrophages What is this structure? Degenerate Neutrophils Abundant amount of keratin with similar structures The structures are ~ 2‐3 micron in size, blue to occasionally dark purple, oval to round in shape and have a thin clear capsule, consistent with fungal organisms The fungi are seen also in neutrophils and free in the background Growth of Microsporum canis was diagnosed on fungal culture • Microsporum canis is a dermatophyte that frequently causes infection of the superficial layers of skin, hair and nails (dermtophytoses). 1,2 • Microsporum canis, Microsporum gypseum and Trichophyton mentagrophytes are the main etiologic agents of clinical dermatophytosis. 3 • Dermatophytes affect the hair shafts, stratum corneum and nails or claws of animals and people. 2 • The typical lesions include focal alopecia, broken hair shafts, crusts scales and erythema on the head, feet and tail of dogs and cats. 1 • The structures seen in this cytology were Microsporum arthroconidia, which should be differentiated from other similar fungal yeast bodies seen in Candidiasis, Histoplasmosis, Aspergillosis/Penicillosis and Sporotrichosis. 2,4 Kerion • Less commonly dermatophytosis causes raised or dermal nodules called kerions or nodular dermatophytosis. 1,3 • These lesions are formed when the infected hair follicle ruptures and both the fungi and keratin spill in the dermis eliciting an intense inflammatory response. 1 • This may develop in any species but is most commonly reported in dogs. 3 • M. canis is the most common cause of kerion in dogs. 3 • Most common locations were the head, neck and limbs. -

Mucormycosis: a Review on Environmental Fungal Spores and Seasonal Variation of Human Disease
Advances in Infectious Diseases, 2012, 2, 76-81 http://dx.doi.org/10.4236/aid.2012.23012 Published Online September 2012 (http://www.SciRP.org/journal/aid) Mucormycosis: A Review on Environmental Fungal Spores and Seasonal Variation of Human Disease Rima I. El-Herte, Tania A. Baban, Souha S. Kanj* Division of Infectious Diseases, Department of Internal Medicine, American University of Beirut Medical Center, Beirut, Lebanon. Email: *[email protected] Received May 1st, 2012; revised June 3rd, 2012; accepted July 5th, 2012 ABSTRACT Mucormycosis is on the rise especially among patients with immunosuppressive conditions. There seems to be more cases seen at the end of summer and towards early autumn. Several studies have attempted to look at the seasonal varia- tions of fungal pathogens in variou indoor and outdoor settings. Only two reports, both from the Middle East, have ad- dressed the relationship of mucormycosis in human disease with climate conditions. In this paper we review, the rela- tionship of indoor and outdoor fungal particulates to the weather conditions and the reported seasonal variation of hu- man cases. Keywords: Mucormycosis; Seasonal Variation; Fungal Air Particulate Concentration; Mucor; Rhizopus; Rhinocerebral 1. Introduction bread, decaying fruits, vegetable matters, crop debris, soil, compost piles, animal excreta, and on excavation and con- Mucormycosis refers to infections caused by molds be- struction sites. Sporangiospores are easily aerosolized, and longing to the order of Mucorales. Members of the fam- are readily dispersed throughout the environment making ily Mucoraceae are the most common cause of mucor- inhalation the major mode of transmission. Published data mycosis in humans. -

Detection of Histoplasma DNA from Tissue Blocks by a Specific
Journal of Fungi Article Detection of Histoplasma DNA from Tissue Blocks by a Specific and a Broad-Range Real-Time PCR: Tools to Elucidate the Epidemiology of Histoplasmosis Dunja Wilmes 1,*, Ilka McCormick-Smith 1, Charlotte Lempp 2 , Ursula Mayer 2 , Arik Bernard Schulze 3 , Dirk Theegarten 4, Sylvia Hartmann 5 and Volker Rickerts 1 1 Reference Laboratory for Cryptococcosis and Uncommon Invasive Fungal Infections, Division for Mycotic and Parasitic Agents and Mycobacteria, Robert Koch Institute, 13353 Berlin, Germany; [email protected] (I.M.-S.); [email protected] (V.R.) 2 Vet Med Labor GmbH, Division of IDEXX Laboratories, 71636 Ludwigsburg, Germany; [email protected] (C.L.); [email protected] (U.M.) 3 Department of Medicine A, Hematology, Oncology and Pulmonary Medicine, University Hospital Muenster, 48149 Muenster, Germany; [email protected] 4 Institute of Pathology, University Hospital Essen, University Duisburg-Essen, 45147 Essen, Germany; [email protected] 5 Senckenberg Institute for Pathology, Johann Wolfgang Goethe University Frankfurt, 60323 Frankfurt am Main, Germany; [email protected] * Correspondence: [email protected]; Tel.: +49-30-187-542-862 Received: 10 November 2020; Accepted: 25 November 2020; Published: 27 November 2020 Abstract: Lack of sensitive diagnostic tests impairs the understanding of the epidemiology of histoplasmosis, a disease whose burden is estimated to be largely underrated. Broad-range PCRs have been applied to identify fungal agents from pathology blocks, but sensitivity is variable. In this study, we compared the results of a specific Histoplasma qPCR (H. qPCR) with the results of a broad-range qPCR (28S qPCR) on formalin-fixed, paraffin-embedded (FFPE) tissue specimens from patients with proven fungal infections (n = 67), histologically suggestive of histoplasmosis (n = 36) and other mycoses (n = 31).