Estimated Burden of Fungal Infections in Oman
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Fungal Infections from Human and Animal Contact
Journal of Patient-Centered Research and Reviews Volume 4 Issue 2 Article 4 4-25-2017 Fungal Infections From Human and Animal Contact Dennis J. Baumgardner Follow this and additional works at: https://aurora.org/jpcrr Part of the Bacterial Infections and Mycoses Commons, Infectious Disease Commons, and the Skin and Connective Tissue Diseases Commons Recommended Citation Baumgardner DJ. Fungal infections from human and animal contact. J Patient Cent Res Rev. 2017;4:78-89. doi: 10.17294/2330-0698.1418 Published quarterly by Midwest-based health system Advocate Aurora Health and indexed in PubMed Central, the Journal of Patient-Centered Research and Reviews (JPCRR) is an open access, peer-reviewed medical journal focused on disseminating scholarly works devoted to improving patient-centered care practices, health outcomes, and the patient experience. REVIEW Fungal Infections From Human and Animal Contact Dennis J. Baumgardner, MD Aurora University of Wisconsin Medical Group, Aurora Health Care, Milwaukee, WI; Department of Family Medicine and Community Health, University of Wisconsin School of Medicine and Public Health, Madison, WI; Center for Urban Population Health, Milwaukee, WI Abstract Fungal infections in humans resulting from human or animal contact are relatively uncommon, but they include a significant proportion of dermatophyte infections. Some of the most commonly encountered diseases of the integument are dermatomycoses. Human or animal contact may be the source of all types of tinea infections, occasional candidal infections, and some other types of superficial or deep fungal infections. This narrative review focuses on the epidemiology, clinical features, diagnosis and treatment of anthropophilic dermatophyte infections primarily found in North America. -

Mucormycosis of the Central Nervous System
Journal of Fungi Review Mucormycosis of the Central Nervous System 1 1,2, , 3, , Amanda Chikley , Ronen Ben-Ami * y and Dimitrios P Kontoyiannis * y 1 Infectious Diseases Unit, Tel Aviv Sourasky Medical Center, Tel Aviv 64239, Israel 2 Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv 64239, Israel 3 Department of Infectious Diseases, The University of Texas, M.D. Anderson Cancer Center, Houston, TX 77030, USA * Correspondence: [email protected] (R.B.-A.); [email protected] (D.P.K.) These authors contribute equally to this paper. y Received: 6 June 2019; Accepted: 7 July 2019; Published: 8 July 2019 Abstract: Mucormycosis involves the central nervous system by direct extension from infected paranasal sinuses or hematogenous dissemination from the lungs. Incidence rates of this rare disease seem to be rising, with a shift from the rhino-orbital-cerebral syndrome typical of patients with diabetes mellitus and ketoacidosis, to disseminated disease in patients with hematological malignancies. We present our current understanding of the pathobiology, clinical features, and diagnostic and treatment strategies of cerebral mucormycosis. Despite advances in imaging and the availability of novel drugs, cerebral mucormycosis continues to be associated with high rates of death and disability. Emerging molecular diagnostics, advances in experimental systems and the establishment of large patient registries are key components of ongoing efforts to provide a timely diagnosis and effective treatment to patients with cerebral mucormycosis. Keywords: central nervous system; mucormycosis; Mucorales; zygomycosis 1. Introduction Mucormycosis is the second most frequent invasive mold disease after aspergillosis [1–3], with rising incidence reported in some countries [4–7]. -

PRIOR AUTHORIZATION CRITERIA BRAND NAME (Generic) SPORANOX ORAL CAPSULES (Itraconazole)
PRIOR AUTHORIZATION CRITERIA BRAND NAME (generic) SPORANOX ORAL CAPSULES (itraconazole) Status: CVS Caremark Criteria Type: Initial Prior Authorization Policy FDA-APPROVED INDICATIONS Sporanox (itraconazole) Capsules are indicated for the treatment of the following fungal infections in immunocompromised and non-immunocompromised patients: 1. Blastomycosis, pulmonary and extrapulmonary 2. Histoplasmosis, including chronic cavitary pulmonary disease and disseminated, non-meningeal histoplasmosis, and 3. Aspergillosis, pulmonary and extrapulmonary, in patients who are intolerant of or who are refractory to amphotericin B therapy. Specimens for fungal cultures and other relevant laboratory studies (wet mount, histopathology, serology) should be obtained before therapy to isolate and identify causative organisms. Therapy may be instituted before the results of the cultures and other laboratory studies are known; however, once these results become available, antiinfective therapy should be adjusted accordingly. Sporanox Capsules are also indicated for the treatment of the following fungal infections in non-immunocompromised patients: 1. Onychomycosis of the toenail, with or without fingernail involvement, due to dermatophytes (tinea unguium), and 2. Onychomycosis of the fingernail due to dermatophytes (tinea unguium). Prior to initiating treatment, appropriate nail specimens for laboratory testing (KOH preparation, fungal culture, or nail biopsy) should be obtained to confirm the diagnosis of onychomycosis. Compendial Uses Coccidioidomycosis2,3 -

Epidemiological Alert: COVID-19 Associated Mucormycosis
Epidemiological Alert: COVID-19 associated Mucormycosis 11 June 2021 Given the potential increase in cases of COVID-19 associated mucormycosis (CAM) in the Region of the Americas, the Pan American Health Organization / World Health Organization (PAHO/WHO) recommends that Member States prepare health services in order to minimize morbidity and mortality due to CAM. Introduction In recent months, an increase in reports of cases of Mucormycosis (previously called zygomycosis) is the term used to name invasive fungal infections (IFI) COVID-19 associated Mucormycosis (CAM) has caused by saprophytic environmental fungi, been observed mainly in people with underlying belonging to the subphylum Mucoromycotina, order diseases, such as diabetes mellitus (DM), diabetic Mucorales. Among the most frequent genera are ketoacidosis, or on steroids. In these patients, the Rhizopus and Mucor; and less frequently Lichtheimia, most frequent clinical manifestation is rhino-orbital Saksenaea, Rhizomucor, Apophysomyces, and Cunninghamela (Nucci M, Engelhardt M, Hamed K. mucormycosis, followed by rhino-orbital-cerebral Mucormycosis in South America: A review of 143 mucormycosis, which present as secondary reported cases. Mycoses. 2019 Sep;62(9):730-738. doi: infections and occur after SARS CoV-2 infection. 1,2 10.1111/myc.12958. Epub 2019 Jul 11. PMID: 31192488; PMCID: PMC6852100). Globally, the highest number of cases has been The infection is acquired by the implantation of the reported in India, where it is estimated that there spores of the fungus in the oral, nasal, and are more than 4,000 people with CAM.3 conjunctival mucosa, by inhalation, or by ingestion of contaminated food, since they quickly colonize foods rich in simple carbohydrates. -

An Aggressive Case of Mucormycosis
Open Access Case Report DOI: 10.7759/cureus.9610 An Aggressive Case of Mucormycosis Donovan Tran 1 , Berndt Schmit 2 1. Diagnostic Radiology, University of Arizona College of Medicine - Tucson, Tucson, USA 2. Radiology, University of Arizona College of Medicine - Tucson, Tucson, USA Corresponding author: Donovan Tran, [email protected] Abstract Mucormycosis is an aggressive fungal disease that can occur in individuals with certain predisposing factors, such as diabetes mellitus and pharmacologic immunosuppression. An astounding aspect of this disease is the speed at which it can spread to surrounding structures once it begins to germinate inside the human body. This case involves a 24-year-old male patient who presented to the emergency room complaining of a headache after a dental procedure who developed fulminant rhinocerebral mucormycosis within days. The objective of this report is to shed light on how fast this disease spreads, discuss current management of rhinocerebral mucormycosis, and illustrate the subtle, but critical radiographic findings to raise clinical awareness for this life-threatening disease. Categories: Emergency Medicine, Radiology, Infectious Disease Keywords: rhinocerebral mucormycosis, mucormycosis, rhizopus, invasive fungal sinusitis, retroantral fat, isavuconazole Introduction We share our world with fungi. They are ubiquitous in nature; current estimates put the number of fungal species to be as high as 5.1 million [1]. As plentiful as they are, only hundreds of these species are pathogenic to humans, collectively killing more than 1.6 million people annually [2]. Common fungi that cause illness are Aspergillus species, Candida albicans, Cryptococcus neoformans, Blastomyces dermatitidis, and Rhizopus species. The term mucormycosis refers to any fungal infection caused by fungi belonging to the Mucorales order [3]. -

Tinea Capitis 2014 L.C
BJD GUIDELINES British Journal of Dermatology British Association of Dermatologists’ guidelines for the management of tinea capitis 2014 L.C. Fuller,1 R.C. Barton,2 M.F. Mohd Mustapa,3 L.E. Proudfoot,4 S.P. Punjabi5 and E.M. Higgins6 1Department of Dermatology, Chelsea & Westminster Hospital, Fulham Road, London SW10 9NH, U.K. 2Department of Microbiology, Leeds General Infirmary, Leeds LS1 3EX, U.K. 3British Association of Dermatologists, Willan House, 4 Fitzroy Square, London W1T 5HQ, U.K. 4St John’s Institute of Dermatology, Guy’s and St Thomas’ NHS Foundation Trust, St Thomas’ Hospital, Westminster Bridge Road, London SE1 7EH, U.K. 5Department of Dermatology, Hammersmith Hospital, 150 Du Cane Road, London W12 0HS, U.K. 6Department of Dermatology, King’s College Hospital, Denmark Hill, London SE5 9RS, U.K. 1.0 Purpose and scope Correspondence Claire Fuller. The overall objective of this guideline is to provide up-to- E-mail: [email protected] date, evidence-based recommendations for the management of tinea capitis. This document aims to update and expand Accepted for publication on the previous guidelines by (i) offering an appraisal of 8 June 2014 all relevant literature since January 1999, focusing on any key developments; (ii) addressing important, practical clini- Funding sources cal questions relating to the primary guideline objective, i.e. None. accurate diagnosis and identification of cases; suitable treat- ment to minimize duration of disease, discomfort and scar- Conflicts of interest ring; and limiting spread among other members of the L.C.F. has received sponsorship to attend conferences from Almirall, Janssen and LEO Pharma (nonspecific); has acted as a consultant for Alliance Pharma (nonspe- community; (iii) providing guideline recommendations and, cific); and has legal representation for L’Oreal U.K. -

Therapies for Common Cutaneous Fungal Infections
MedicineToday 2014; 15(6): 35-47 PEER REVIEWED FEATURE 2 CPD POINTS Therapies for common cutaneous fungal infections KENG-EE THAI MB BS(Hons), BMedSci(Hons), FACD Key points A practical approach to the diagnosis and treatment of common fungal • Fungal infection should infections of the skin and hair is provided. Topical antifungal therapies always be in the differential are effective and usually used as first-line therapy, with oral antifungals diagnosis of any scaly rash. being saved for recalcitrant infections. Treatment should be for several • Topical antifungal agents are typically adequate treatment weeks at least. for simple tinea. • Oral antifungal therapy may inea and yeast infections are among the dermatophytoses (tinea) and yeast infections be required for extensive most common diagnoses found in general and their differential diagnoses and treatments disease, fungal folliculitis and practice and dermatology. Although are then discussed (Table). tinea involving the face, hair- antifungal therapies are effective in these bearing areas, palms and T infections, an accurate diagnosis is required to ANTIFUNGAL THERAPIES soles. avoid misuse of these or other topical agents. Topical antifungal preparations are the most • Tinea should be suspected if Furthermore, subsequent active prevention is commonly prescribed agents for dermatomy- there is unilateral hand just as important as the initial treatment of the coses, with systemic agents being used for dermatitis and rash on both fungal infection. complex, widespread tinea or when topical agents feet – ‘one hand and two feet’ This article provides a practical approach fail for tinea or yeast infections. The pharmacol- involvement. to antifungal therapy for common fungal infec- ogy of the systemic agents is discussed first here. -
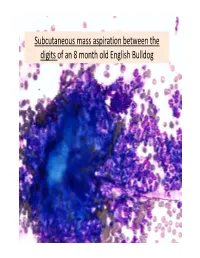
Subcutaneous Mass Aspiration Between the Digits of an 8 Month Old English Bulldog Marked Inflammation: Neutrophils & Macrophages
Subcutaneous mass aspiration between the digits of an 8 month old English Bulldog Marked inflammation: neutrophils & macrophages Hair fragment Macrophages What is this structure? Degenerate Neutrophils Abundant amount of keratin with similar structures The structures are ~ 2‐3 micron in size, blue to occasionally dark purple, oval to round in shape and have a thin clear capsule, consistent with fungal organisms The fungi are seen also in neutrophils and free in the background Growth of Microsporum canis was diagnosed on fungal culture • Microsporum canis is a dermatophyte that frequently causes infection of the superficial layers of skin, hair and nails (dermtophytoses). 1,2 • Microsporum canis, Microsporum gypseum and Trichophyton mentagrophytes are the main etiologic agents of clinical dermatophytosis. 3 • Dermatophytes affect the hair shafts, stratum corneum and nails or claws of animals and people. 2 • The typical lesions include focal alopecia, broken hair shafts, crusts scales and erythema on the head, feet and tail of dogs and cats. 1 • The structures seen in this cytology were Microsporum arthroconidia, which should be differentiated from other similar fungal yeast bodies seen in Candidiasis, Histoplasmosis, Aspergillosis/Penicillosis and Sporotrichosis. 2,4 Kerion • Less commonly dermatophytosis causes raised or dermal nodules called kerions or nodular dermatophytosis. 1,3 • These lesions are formed when the infected hair follicle ruptures and both the fungi and keratin spill in the dermis eliciting an intense inflammatory response. 1 • This may develop in any species but is most commonly reported in dogs. 3 • M. canis is the most common cause of kerion in dogs. 3 • Most common locations were the head, neck and limbs. -

Therapies for Common Cutaneous Fungal Infections
MedicineToday 2014; 15(6): 35-47 PEER REVIEWED FEATURE 2 CPD POINTS Therapies for common cutaneous fungal infections KENG-EE THAI MB BS(Hons), BMedSci(Hons), FACD Key points A practical approach to the diagnosis and treatment of common fungal • Fungal infection should infections of the skin and hair is provided. Topical antifungal therapies always be in the differential are effective and usually used as first-line therapy, with oral antifungals diagnosis of any scaly rash. being saved for recalcitrant infections. Treatment should be for several • Topical antifungal agents are typically adequate treatment weeks at least. for simple tinea. • Oral antifungal therapy may inea and yeast infections are among the dermatophytoses (tinea) and yeast infections be required for extensive most common diagnoses found in general and their differential diagnoses and treatments disease, fungal folliculitis and practice and dermatology. Although are then discussed (Table). tinea involving the face, hair- antifungal therapies are effective in these bearing areas, palms and T infections, an accurate diagnosis is required to ANTIFUNGAL THERAPIES soles. avoid misuse of these or other topical agents. Topical antifungal preparations are the most • Tinea should be suspected if Furthermore, subsequent active prevention is commonly prescribed agents for dermatomy- there is unilateral hand just as important as the initial treatment of the coses, with systemic agents being used for dermatitis and rash on both fungal infection. complex, widespread tinea or when topical agents feet – ‘one hand and two feet’ This article provides a practical approach fail for tinea or yeast infections. The pharmacol- involvement. to antifungal therapy for common fungal infec- ogy of the systemic agents is discussed first here. -

Clinical Features of Pulmonary Mucormycosis in Patients with Different Immune Status
5052 Original Article Clinical features of pulmonary mucormycosis in patients with different immune status Min Peng1#, Hua Meng2#, Yinghao Sun3, Yu Xiao4, Hong Zhang1, Ke Lv2, Baiqiang Cai1 1Division of Pulmonary and Critical Care Medicine, 2Department of Ultrasound, 3Department of Internal Medicine, 4Department of Pathology, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing 100730, China Contributions: (I) Conception and design: M Peng, H Zhang, K Lv; (II) Administrative support: None; (III) Provision of study materials or patients: M Peng, Y Xiao, H Zhang; (IV) Collection and assembly of data: H Meng; Y Sun; (V) Data analysis and interpretation: M Peng, H Zhang; (VI) Manuscript writing: All authors; (VII) Final approval of manuscript: All authors. #These authors contributed equally to this work. Correspondence to: Dr. Hong Zhang; Dr. Ke lv. No.1 Shuaifuyuan, Dongcheng District, Beijing 100730, China. Email: [email protected]; [email protected]. Background: Pulmonary mucormycosis (PM) is a relatively rare but often fatal and rapidly progressive disease. Most studies of PM are case reports or case series with limited numbers of patients, and focus on immunocompromised patients. We investigated the clinical manifestations, imaging features, treatment, and outcomes of patients with PM with a focus on the difference in clinical manifestations between patients with different immune status. Methods: Clinical records, laboratory results, and computed tomography scans of 24 patients with proven or probable PM from January 2005 to December 2018 in Peking Union Medical College Hospital were retrospectively analyzed. Results: Ten female and 14 male patients were included (median age, 43.5 years; range, 13–64 years). -

Concomitant Mucormycosis with Aspergillosis in Patients with Uncontrolled Diabetes
DOI: 10.7860/JCDR/2021/47912.14507 Case Series Concomitant Mucormycosis with Aspergillosis in Patients with Uncontrolled Diabetes Microbiology Section Microbiology Mellitus: A Case Series ARPANA SINGH1, AROOP MOHANTY2, SHWETA JHA3, PRATIMA GUPTA4, NEELAM KAISTHA5 ABSTRACT Fungal infections are life threatening especially in presence of immunosuppression or uncontrolled diabetes mellitus mainly due to their invasive potential. Mucormycosis of the oculo-rhino-cerebral region is an opportunistic, aggressive, fatal and rapidly spreading infection caused by organisms belonging to Mucorales order and class Zygomycetes. The organisms associated are ubiquitous. Aspergillosis is a common clinical condition caused by the Aspergillus species, most often by Aspergillus fumigatus (A. fumigatus). Both fungi have a predilection for the immunosuppressive conditions, with uncontrolled diabetes and malignancy being the most common among them. Mucormycosis is caused by environmental spores which get access into the body through the lungs and cause various systemic manifestations like rhino-cerebral mucormycosis. Here, a case series of such concomitant infections of Aspergillus and Mucor spp from Rishikesh, Uttarakhand, India is reported. Keywords: Diabetes, Fungal infection, Invasive mycoses, Rhinosinusitis INTRODUCTION Case 2 Mucorales are the universally distributed saprophytes causing A 60-year-old female patient presented with complaints of aggressive and opportunistic infection. They are angio-invasive fever for three days and ptosis for one day. She was a known in nature. Aspergillosis is the clinical condition caused by the case of Type 2 Diabetes Mellitus (T2DM) and hypertension for Aspergillus species most often A. fumigatus [1]. It proves to be the past seven years but was on irregular drug metformin and fatal, if it infects secondarily to the brain. -

A Review of Prolonged Post-COVID-19 Symptoms and Their Implications on Dental Management
International Journal of Environmental Research and Public Health Review A Review of Prolonged Post-COVID-19 Symptoms and Their Implications on Dental Management Trishnika Chakraborty 1,2 , Rizwana Fathima Jamal 3 , Gopi Battineni 4 , Kavalipurapu Venkata Teja 5 , Carlos Miguel Marto 6,7,8,9 and Gianrico Spagnuolo 10,11,* 1 Department of Conservative Dentistry and Endodontics, Chaudhary Charan Singh University, Meerut, Uttar Pradesh 250001, India; [email protected] 2 Department of Health System Management, Ben-Gurion University of Negev, Beer-Sheva 8410501, Israel 3 Department of Oral and Maxillofacial Surgery, Chettinad Dental College and Research Institute, Kancheepuram, Tamil Nadu 603103, India; [email protected] 4 Telemedicine and Tele Pharmacy Center, School Medicinal and Health Products Sciences, University of Camerino, 62032 Camerino, Italy; [email protected] 5 Department of Conservative Dentistry & Endodontics, Saveetha Dental College & Hospitals, Saveetha Institute of Medical & Technical Sciences, Saveetha University, Chennai, Tamil Nadu 600077, India; [email protected] 6 Faculty of Medicine, Institute of Experimental Pathology, University of Coimbra, 3004-531 Coimbra, Portugal; [email protected] 7 Faculty of Medicine, Coimbra Institute for Clinical and Biomedical Research (iCBR), University of Coimbra, Area of Environment Genetics and Oncobiology (CIMAGO), 3000-548 Coimbra, Portugal 8 Centre for Innovative Biomedicine and Biotechnology (CIBB), University of Coimbra, 3004-504 Coimbra, Portugal 9 Clinical Academic Center of Coimbra (CACC), 3004-531 Coimbra, Portugal 10 Department of Neurosciences, Reproductive and Odontostomatological Sciences, University of Naples “Federico II”, 80131 Napoli, Italy Citation: Chakraborty, T.; Jamal, R.F.; 11 Institute of Dentistry, I. M. Sechenov First Moscow State Medical University, 119435 Moscow, Russia Battineni, G.; Teja, K.V.; Marto, C.M.; * Correspondence: [email protected] Spagnuolo, G.