DRUG NAME: Idarubicin
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
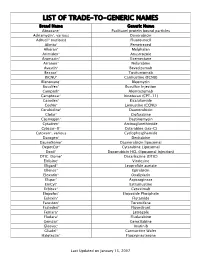
Trade-To-Generic Names
LIST OF TRADE-TO-GENERIC NAMES Brand Name Generic Name Abraxane® Paclitaxel protein bound particles Adriamycin®, various Doxorubicin Adrucil® (various) Fluorouracil Alimta® Pemetrexed Alkeran® Melphalan Arimidex® Anastrozole Aromasin® Exemestane Arranon® Nelarabine Avastin® Bevacizumab Bexxar® Tositumomab BiCNU® Carmustine (BCNU) Blenoxane® Bleomycin Busulfex® Busulfan Injection Campath® Alemtuzumab Camptosar® Irinotecan (CPT-11) Casodex® Bicalutamide CeeNu® Lomustine (CCNU) Cerubidine® Daunorubicin Clolar® Clofarabine Cosmegen® Dactinomycin Cytadren® Aminoglutethimide Cytosar-U® Cytarabine (ara-C) Cytoxan®, various Cyclophosphamide Dacogen® Decitabine DaunoXome® Daunorubicin liposomal DepotCyt® Cytarabine Liposomal Doxil® Doxorubicin HCL (liposomal injection) DTIC-Dome® Dacarbazine (DTIC) Eldisine® Vindesine Eligard® Leuprolide acetate Ellence® Epirubicin Eloxatin® Oxaliplatin Elspar® Asparaginase EmCyt® Estramustine Erbitux® Cetuximab Etopofos® Etoposide Phosphate Eulexin® Flutamide Fareston® Toremifene Faslodex® Fluvestrant Femara® Letrozole Fludara® Fludarabine Gemzar® Gemcitabine Gleevec® Imatinib Gliadel® Carmustine Wafer Halotestin® Fluoxymesterone Last Updated on January 15, 2007 Brand Name Generic Name Herceptin® Trastuzumab Hexalen® Altretamine Hycamtin® Topotecan Hydrea® Hydroxyurea Idamycin® Idarubicin Ifex® Ifosfamide Intron A® Interferon alfa-2b Iressa® Gefitinib Leukeran® Chlorambucil Leukine® Sargramostim Leustatin® Cladribine Lupron depot® Leuprolide acetate depot Lupron® Leuprolide acetate Matulane® Procarbazine Megace® -

Idamycin PFS® Idarubicin Hydrochloride Injection
Idamycin PFS® idarubicin hydrochloride injection Rx only FOR INTRAVENOUS USE ONLY WARNINGS 1. IDAMYCIN PFS Injection should be given slowly into a freely flowing intravenous infusion. It must never be given intramuscularly or subcutaneously. Severe local tissue necrosis can occur if there is extravasation during administration. 2. As is the case with other anthracyclines the use of IDAMYCIN PFS can cause myocardial toxicity leading to congestive heart failure. Cardiac toxicity is more common in patients who have received prior anthracyclines or who have pre- existing cardiac disease. 3. As is usual with antileukemic agents, severe myelosuppression occurs when IDAMYCIN PFS is used at effective therapeutic doses. 4. It is recommended that IDAMYCIN PFS be administered only under the supervision of a physician who is experienced in leukemia chemotherapy and in facilities with laboratory and supportive resources adequate to monitor drug tolerance and protect and maintain a patient compromised by drug toxicity. The physician and institution must be capable of responding rapidly and completely to severe hemorrhagic conditions and/or overwhelming infection. 5. Dosage should be reduced in patients with impaired hepatic or renal function. (See DOSAGE AND ADMINISTRATION.) DESCRIPTION IDAMYCIN PFS Injection contains idarubicin hydrochloride and is a sterile, semi- synthetic, preservative-free solution (PFS) antineoplastic anthracycline for intravenous use. Chemically, idarubicin hydrochloride is 5, 12-Naphthacenedione, 9-acetyl-7-[(3- amino-2,3,6-trideoxy-α-L-lyxo-hexopyranosyl)oxy]-7,8,9,10-tetrahydro-6,9,11- trihydroxyhydrochloride, (7S-cis). The structural formula is as follows: C26 H27 NO9 .HCL M.W. 533.96 1 Reference ID: 3668307 IDAMYCIN PFS is a sterile, red-orange, isotonic parenteral preservative-free solution, available in 5 mL (5 mg), 10 mL (10 mg) and 20 mL (20 mg) single-use-only vials. -

AHFS Pharmacologic-Therapeutic Classification System
AHFS Pharmacologic-Therapeutic Classification System Abacavir 48:24 - Mucolytic Agents - 382638 8:18.08.20 - HIV Nucleoside and Nucleotide Reverse Acitretin 84:92 - Skin and Mucous Membrane Agents, Abaloparatide 68:24.08 - Parathyroid Agents - 317036 Aclidinium Abatacept 12:08.08 - Antimuscarinics/Antispasmodics - 313022 92:36 - Disease-modifying Antirheumatic Drugs - Acrivastine 92:20 - Immunomodulatory Agents - 306003 4:08 - Second Generation Antihistamines - 394040 Abciximab 48:04.08 - Second Generation Antihistamines - 394040 20:12.18 - Platelet-aggregation Inhibitors - 395014 Acyclovir Abemaciclib 8:18.32 - Nucleosides and Nucleotides - 381045 10:00 - Antineoplastic Agents - 317058 84:04.06 - Antivirals - 381036 Abiraterone Adalimumab; -adaz 10:00 - Antineoplastic Agents - 311027 92:36 - Disease-modifying Antirheumatic Drugs - AbobotulinumtoxinA 56:92 - GI Drugs, Miscellaneous - 302046 92:20 - Immunomodulatory Agents - 302046 92:92 - Other Miscellaneous Therapeutic Agents - 12:20.92 - Skeletal Muscle Relaxants, Miscellaneous - Adapalene 84:92 - Skin and Mucous Membrane Agents, Acalabrutinib 10:00 - Antineoplastic Agents - 317059 Adefovir Acamprosate 8:18.32 - Nucleosides and Nucleotides - 302036 28:92 - Central Nervous System Agents, Adenosine 24:04.04.24 - Class IV Antiarrhythmics - 304010 Acarbose Adenovirus Vaccine Live Oral 68:20.02 - alpha-Glucosidase Inhibitors - 396015 80:12 - Vaccines - 315016 Acebutolol Ado-Trastuzumab 24:24 - beta-Adrenergic Blocking Agents - 387003 10:00 - Antineoplastic Agents - 313041 12:16.08.08 - Selective -

BC Cancer Protocol Summary for Treatment of Lymphoma with Dose- Adjusted Etoposide, Doxorubicin, Vincristine, Cyclophosphamide
BC Cancer Protocol Summary for Treatment of Lymphoma with Dose- Adjusted Etoposide, DOXOrubicin, vinCRIStine, Cyclophosphamide, predniSONE and riTUXimab with Intrathecal Methotrexate Protocol Code LYEPOCHR Tumour Group Lymphoma Contact Physician Dr. Laurie Sehn Dr. Kerry Savage ELIGIBILITY: One of the following lymphomas: . Patients with an aggressive B-cell lymphoma and the presence of a dual translocation of MYC and BCL2 (i.e., double-hit lymphoma). Histologies may include DLBCL, transformed lymphoma, unclassifiable lymphoma, and intermediate grade lymphoma, not otherwise specified (NOS). Patients with Burkitt lymphoma, who are not candidates for CODOXM/IVACR (such as those over the age of 65 years, or with significant co-morbidities) . Primary mediastinal B-cell lymphoma Ensure patient has central line EXCLUSIONS: . Cardiac dysfunction that would preclude the use of an anthracycline. TESTS: . Baseline (required before first treatment): CBC and diff, platelets, BUN, creatinine, bilirubin. ALT, LDH, uric acid . Baseline (required, but results do not have to be available to proceed with first treatment): results must be checked before proceeding with cycle 2): HBsAg, HBcoreAb, . Baseline (optional, results do not have to be available to proceed with first treatment): HCAb, HIV . Day 1 of each cycle: CBC and diff, platelets, (and serum bilirubin if elevated at baseline; serum bilirubin does not need to be requested before each treatment, after it has returned to normal), urinalysis for microscopic hematuria (optional) . Days 2 and 5 of each cycle (or days of intrathecal treatment): CBC and diff, platelets, PTT, INR . For patients on cyclophosphamide doses greater than 2000 mg: Daily urine dipstick for blood starting on day cyclophosphamide is given. -

A Comparison of Early Intensive Methotrexate/Mercaptopurine With
Leukemia (2001) 15, 1038–1045 2001 Nature Publishing Group All rights reserved 0887-6924/01 $15.00 www.nature.com/leu A comparison of early intensive methotrexate/mercaptopurine with early intensive alternating combination chemotherapy for high-risk B-precursor acute lymphoblastic leukemia: a Pediatric Oncology Group phase III randomized trial SJ Lauer1, JJ Shuster2, DH Mahoney Jr3, N Winick4, S Toledano5, L Munoz5, G Kiefer6, JD Pullen7, CP Steuber3 and BM Camitta6 1Emory University School of Medicine, Atlanta, GA; 2Pediatric Oncology Group Statistical Office and Department of Statistics, University of Florida, Gainesville, FL; 3Texas Children’s Cancer Center, Baylor College of Medicine, Houston, TX; 4University of Texas Southwestern Medical Center, Dallas, TX; 5University Miami School of Medicine, Miami, FL; 6Midwest Children’s Cancer Center, Milwaukee, WI; and 7University of Mississippi Medical Center Children’s Hospital, Jackson, MS, USA A prospective, randomized multicenter study was performed to to their risk for relapse and treatment strategies designed to evaluate the relative efficacy of two different concepts for early improve event-free survival (EFS). intensive therapy in a randomized trial of children with B-pre- cursor acute lymphoblastic leukemia (ALL) at high risk (HR) for It is believed that the leading causes of relapse in children relapse. Four hundred and ninety eligible children with HR-ALL with higher risk ALL (HR-ALL) are inadequate cell kill and were randomized on the Pediatric Oncology Group (POG) 9006 emergence of drug-resistant clones. Clinical trials using early phase III trial between 7 January 1991 and 12 January 1994. intensive myelosuppressive combination chemotherapy as After prednisone (PDN), vincristine (VCR), asparaginase (ASP) post-induction consolidation were designed to maximize cell and daunorubicin (DNR) induction, 470 patients received either 2 kill and address drug resistance. -

Idarubicin.Pdf
PRODUCT MONOGRAPH INCLUDING PATIENT MEDICATION INFORMATION PrIDARUBICIN idarubicin hydrochloride injection Preservative Free Solution, 1 mg/mL, intravenous injection Antineoplastic Agent Pfizer Canada ULC Date of Initial Authorization: 17,300 Trans-Canada Highway July 30, 2013 Kirkland, Quebec, H9J 2M5 Date of Revision: September 14, 2021 Submission Control Number: 255782 ©Pfizer Canada ULC, 2021 IDARUBICIN (idarubicin hydrochloride injection) Page 1 of 31 RECENT MAJOR LABEL CHANGES 7 Warnings and Precautions, Cardiovascular JA/2021 TABLE OF CONTENTS Sections or subsections that are not applicable at the time of authorization are not listed. RECENT MAJOR LABEL CHANGES ..........................................................................................2 TABLE OF CONTENTS ............................................................................................................2 1 INDICATIONS.............................................................................................................4 1.1 Pediatrics .................................................................................................................4 1.2 Geriatrics..................................................................................................................4 2 CONTRAINDICATIONS................................................................................................4 3 SERIOUS WARNINGS AND PRECAUTIONS BOX ...........................................................5 4 DOSAGE AND ADMINISTRATION................................................................................5 -

Incidence of Differentiation Syndrome Associated with Treatment
Journal of Clinical Medicine Review Incidence of Differentiation Syndrome Associated with Treatment Regimens in Acute Myeloid Leukemia: A Systematic Review of the Literature Lucia Gasparovic 1, Stefan Weiler 1,2, Lukas Higi 1 and Andrea M. Burden 1,* 1 Institute of Pharmaceutical Sciences, Department of Chemistry and Applied Biosciences, ETH Zurich, 8093 Zurich, Switzerland; [email protected] (L.G.); [email protected] (S.W.); [email protected] (L.H.) 2 National Poisons Information Centre, Tox Info Suisse, Associated Institute of the University of Zurich, 8032 Zurich, Switzerland * Correspondence: [email protected]; Tel.: +41-76-685-22-56 Received: 30 August 2020; Accepted: 14 October 2020; Published: 18 October 2020 Abstract: Differentiation syndrome (DS) is a potentially fatal adverse drug reaction caused by the so-called differentiating agents such as all-trans retinoic acid (ATRA) and arsenic trioxide (ATO), used for remission induction in the treatment of the M3 subtype of acute myeloid leukemia (AML), acute promyelocytic leukemia (APL). However, recent DS reports in trials of isocitrate dehydrogenase (IDH)-inhibitor drugs in patients with IDH-mutated AML have raised concerns. Given the limited knowledge of the incidence of DS with differentiating agents, we conducted a systematic literature review of clinical trials with reports of DS to provide a comprehensive overview of the medications associated with DS. In particular, we focused on the incidence of DS reported among the IDH-inhibitors, compared to existing ATRA and ATO therapies. We identified 44 published articles, encompassing 39 clinical trials, including 6949 patients. Overall, the cumulative incidence of DS across all treatment regimens was 17.7%. -

Summary Attachment for Eudract
Avenue E. Mounier 83/11 1200 Brussels Belgium Tel: +32 2 774 1611 Email: [email protected] www.eortc.org Summary Attachment for EudraCT Name of Individual study Table Referring to Part of the Dossier (For National Authority Use Sponsor/Company: Only) EORTC Name of the Volume: finished product Name of Active Page Ingredients: Clofarabine Cytarabine Idarubicin Title of the Study Clofarabine in combination with a standard remissioninduction regimen (AraC and idarubicin) in patients 18-60 years old with previously untreated intermediate and bad risk acute myelogenous leukemia (AML) or high risk myelodysplasia (MDS) : a phase I-II study of the EORTC-LG and GIMEMA (AML-14A trial) Investigators & Study Centers Number of Country City patients Belgium 4 101.Hopital Jules Bordet (BE) Brussels 1 109.A.Z. St Jan (BE) Brugge 3 Italy 28 3931.Tor Vergata Roma (IT) Roma 12 733.La Sapienza Ematologia (IT) Roma 16 Netherlands 43 304.RU Nijmegen (NL) Nijmegen 24 310.Univ Med Ctr Leiden (NL) Leiden 13 22.J Bosch 'S Hertogenb (NL) 's-Hertogenbosch 6 Grand Total 75 ST-006-AF-01 Page 1 of 4 Template version 2 Short Study Report for Health Authorities EORTC Name of Individual study Table Referring to Part of the Dossier (For National Authority Use Sponsor/Company: Only) EORTC Name of the Volume: finished product Name of Active Page Ingredients: Clofarabine Cytarabine Idarubicin Publication Willemze R, Suciu S, Muus P, Halkes CJ, Meloni G, Meert L, Karrasch M, Rapion J, (reference) Vignetti M, Amadori S, de Witte T, Marie JP.Clofarabine in combination with a standard remission induction regimen (cytosine arabinoside and idarubicin) in patients with previously untreated intermediate and bad-risk acute myelogenous leukemia (AML) or high-risk myelodysplastic syndrome (HR-MDS): phase I results of an ongoing phase I/II study of the leukemia groups of EORTC and GIMEMA (EORTC GIMEMA 06061/AML-14A trial). -

Daunorubicin Hydrochloride Injection Hikma Pharmaceuticals USA Inc
DAUNORUBICIN HYDROCHLORIDE- daunorubicin hydrochloride injection Hikma Pharmaceuticals USA Inc. ---------- DAUNORUBICIN HYDROCHLORIDE INJECTION Rx ONLY WARNINGS 1.Daunorubicin Hydrochloride Injection must be given into a rapidly flowing intravenous infusion. It must never be given by the intramuscular or subcutaneous route. Severe local tissue necrosis will occur if there is extravasation during administration. 2.Myocardial toxicity manifested in its most severe form by potentially fatal congestive heart failure may occur either during therapy or months to years after termination of therapy. The incidence of myocardial toxicity increases after a total cumulative dose exceeding 400 to 550 mg/m2 in adults, 300 mg/m2 in children more than 2 years of age, or 10 mg/kg in children less than 2 years of age. 3.Severe myelosuppression occurs when used in therapeutic doses; this may lead to infection or hemorrhage. 4.It is recommended that daunorubicin hydrochloride be administered only by physicians who are experienced in leukemia chemotherapy and in facilities with laboratory and supportive resources adequate to monitor drug tolerance and protect and maintain a patient compromised by drug toxicity. The physician and institution must be capable of responding rapidly and completely to severe hemorrhagic conditions and/or overwhelming infection. 5.Dosage should be reduced in patients with impaired hepatic or renal function. DESCRIPTION Daunorubicin hydrochloride is the hydrochloride salt of an anthracycline cytotoxic antibiotic produced by a strain of Streptomyces coeruleorubidus. It is provided as a deep red sterile liquid in vials for intravenous administration only. Each mL contains 5 mg daunorubicin (equivalent to 5.34 mg of daunorubicin hydrochloride), 9 mg sodium chloride; sodium hydroxide and/or hydrochloric acid (to adjust pH), and water for injection, q.s. -

BC Cancer Benefit Drug List September 2021
Page 1 of 65 BC Cancer Benefit Drug List September 2021 DEFINITIONS Class I Reimbursed for active cancer or approved treatment or approved indication only. Reimbursed for approved indications only. Completion of the BC Cancer Compassionate Access Program Application (formerly Undesignated Indication Form) is necessary to Restricted Funding (R) provide the appropriate clinical information for each patient. NOTES 1. BC Cancer will reimburse, to the Communities Oncology Network hospital pharmacy, the actual acquisition cost of a Benefit Drug, up to the maximum price as determined by BC Cancer, based on the current brand and contract price. Please contact the OSCAR Hotline at 1-888-355-0355 if more information is required. 2. Not Otherwise Specified (NOS) code only applicable to Class I drugs where indicated. 3. Intrahepatic use of chemotherapy drugs is not reimbursable unless specified. 4. For queries regarding other indications not specified, please contact the BC Cancer Compassionate Access Program Office at 604.877.6000 x 6277 or [email protected] DOSAGE TUMOUR PROTOCOL DRUG APPROVED INDICATIONS CLASS NOTES FORM SITE CODES Therapy for Metastatic Castration-Sensitive Prostate Cancer using abiraterone tablet Genitourinary UGUMCSPABI* R Abiraterone and Prednisone Palliative Therapy for Metastatic Castration Resistant Prostate Cancer abiraterone tablet Genitourinary UGUPABI R Using Abiraterone and prednisone acitretin capsule Lymphoma reversal of early dysplastic and neoplastic stem changes LYNOS I first-line treatment of epidermal -

Rasburicase (Elitek)
Rasburicase (Elitek) ELITEK™ (rasburicase) BOXED WARNINGS Anaphylaxis ELITEK may cause severe hypersensitivity reactions including anaphylaxis. ELITEK should be immediately and permanently discontinued in any patient developing clinical evidence of a serious hypersensitivity reaction (see WARNINGS, Anaphylaxis and ADVERSE REACTIONS, Immunogenicity). Hemolysis ELITEK administered to patients with glucose-6-phosphate dehydrogenase (G6PD) deficiency can cause severe hemolysis. ELITEK administration should be immediately and permanently discontinued in any patient developing hemolysis. It is recommended that patients at higher risk for G6PD deficiency (e.g., patients of African or Mediterranean ancestry) be screened prior to starting ELITEK therapy (see CONTRAINDICATIONS and WARNINGS, Hemolysis). Methemoglobinemia ELITEK use has been associated with Methemoglobinemia. ELITEK administration should be immediately and permanently discontinued in any patient identified as having developed methemoglobinemia (see WARNINGS, Methemoglobinemia). Interference with Uric Acid Measurements ELITEK will cause enzymatic degradation of the uric acid within blood samples left at room temperature, resulting in spuriously low uric acid levels. To ensure accurate measurements, blood must be collected into pre-chilled tubes containing heparin anticoagulent and immediately immersed and maintained in an ice water bath; plasma samples must be assayed within 4 hours of sample collection (see PRECAUTIONS, Laboratory Test Interactions). DESCRIPTION ELITEK (rasburicase) is a recombinant urate-oxidase enzyme produced by a genetically modified Saccharomyces cerevisiae strain. The cDNA coding for rasburicase was cloned from a strain of Aspergillus flavus. Rasburicase is a tetrameric protein with identical subunits of a molecular mass of about 34 kDa. The molecular formula of the monomer is C1523 H2383 N417 O462 S7. The monomer, made up of a single 301 amino acid polypeptide chain, has no intra- or inter-disulfide bridges and is N-terminal acetylated. -

How I Treat How I Treat Hyperleukocytosis in Acute Myeloid Leukemia
From www.bloodjournal.org by guest on January 2, 2016. For personal use only. How I Treat How I treat hyperleukocytosis in acute myeloid leukemia Christoph R¨olligand Gerhard Ehninger Medizinische Klinik und Poliklinik I, Universit¨atsklinikumder Technischen Universit¨at Dresden, Germany Hyperleukocytosis (HL) per se is a labora- chronic leukemias, and particularly leuko- lactate dehydrogenase as an indicator for tory abnormality, commonly defined by stasis occurs more often in acute myeloid high proliferation are part of prognostic a white blood cell count >100 000/mL, leukemia (AML) for several reasons. Only scores guiding risk-adapted consolidation caused by leukemic cell proliferation. Not a small proportion of AML patients present strategies, HL at initial diagnosis must be the high blood count itself, but complica- with HL, but these patients have a partic- considered a hematologic emergency and tions such as leukostasis, tumor lysis syn- ularly dismal prognosis because of (1) a requires rapid action of the admitting drome, and disseminated intravascular higher risk of early death resulting from physician in order to prevent early death. coagulation put the patient at risk and HL complications; and (2) a higher proba- (Blood. 2015;125(21):3246-3252) require therapeutic intervention. The risk bility of relapse and death in the long run. of complications is higher in acute than in Whereas initial high blood counts and high Incidence and pathophysiology In untreated acute myeloid leukemia (AML), ;5% to 20% of patients factor VII.18 TLS may occur as a result of spontaneous or treatment- present with hyperleukocytosis (HL).1-10 In a patient with HL, under- induced cell death.