Supplement of Tropospheric Sources and Sinks of Gas-Phase Acids in the Colorado Front Range
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Development Team
Paper No: 16 Environmental Chemistry Module: 01 Environmental Concentration Units Development Team Prof. R.K. Kohli Principal Investigator & Prof. V.K. Garg & Prof. Ashok Dhawan Co- Principal Investigator Central University of Punjab, Bathinda Prof. K.S. Gupta Paper Coordinator University of Rajasthan, Jaipur Prof. K.S. Gupta Content Writer University of Rajasthan, Jaipur Content Reviewer Dr. V.K. Garg Central University of Punjab, Bathinda Anchor Institute Central University of Punjab 1 Environmental Chemistry Environmental Environmental Concentration Units Sciences Description of Module Subject Name Environmental Sciences Paper Name Environmental Chemistry Module Name/Title Environmental Concentration Units Module Id EVS/EC-XVI/01 Pre-requisites A basic knowledge of concentration units 1. To define exponents, prefixes and symbols based on SI units 2. To define molarity and molality 3. To define number density and mixing ratio 4. To define parts –per notation by volume Objectives 5. To define parts-per notation by mass by mass 6. To define mass by volume unit for trace gases in air 7. To define mass by volume unit for aqueous media 8. To convert one unit into another Keywords Environmental concentrations, parts- per notations, ppm, ppb, ppt, partial pressure 2 Environmental Chemistry Environmental Environmental Concentration Units Sciences Module 1: Environmental Concentration Units Contents 1. Introduction 2. Exponents 3. Environmental Concentration Units 4. Molarity, mol/L 5. Molality, mol/kg 6. Number Density (n) 7. Mixing Ratio 8. Parts-Per Notation by Volume 9. ppmv, ppbv and pptv 10. Parts-Per Notation by Mass by Mass. 11. Mass by Volume Unit for Trace Gases in Air: Microgram per Cubic Meter, µg/m3 12. -

The Vertical Distribution of Soluble Gases in the Troposphere
UC Irvine UC Irvine Previously Published Works Title The vertical distribution of soluble gases in the troposphere Permalink https://escholarship.org/uc/item/7gc9g430 Journal Geophysical Research Letters, 2(8) ISSN 0094-8276 Authors Stedman, DH Chameides, WL Cicerone, RJ Publication Date 1975 DOI 10.1029/GL002i008p00333 License https://creativecommons.org/licenses/by/4.0/ 4.0 Peer reviewed eScholarship.org Powered by the California Digital Library University of California Vol. 2 No. 8 GEOPHYSICAL RESEARCHLETTERS August 1975 THE VERTICAL DISTRIBUTION OF SOLUBLE GASES IN THE TROPOSPHERE D. H. Stedman, W. L. Chameides, and R. J. Cicerone Space Physics Research Laboratory Department of Atmospheric and Oceanic Science The University of Michigan, Ann Arbor, Michigan 48105 Abstrg.ct. The thermodynamic properties of sev- Their arg:uments were based on the existence of an eral water-soluble gases are reviewed to determine HC1-H20 d•mer, but we consider the conclusions in- the likely effect of the atmospheric water cycle dependeht of the existence of dimers. For in- on their vertical profiles. We find that gaseous stance, Chameides[1975] has argued that HNO3 HC1, HN03, and HBr are sufficiently soluble in should follow the water-vapor mixing ratio gradi- water to suggest that their vertical profiles in ent, based on the high solubility of the gas in the troposphere have a similar shape to that of liquid water. In this work, we generalize the water va•or. Thuswe predict that HC1,HN03, and moredetailed argumentsof Chameides[1975] and HBr exhibit a steep negative gradient with alti- suggest which species should exhibit this nega- tude roughly equal to the altitude gradient of tive gradient and which should not. -

Package 'Marelac'
Package ‘marelac’ February 20, 2020 Version 2.1.10 Title Tools for Aquatic Sciences Author Karline Soetaert [aut, cre], Thomas Petzoldt [aut], Filip Meysman [cph], Lorenz Meire [cph] Maintainer Karline Soetaert <[email protected]> Depends R (>= 3.2), shape Imports stats, seacarb Description Datasets, constants, conversion factors, and utilities for 'MArine', 'Riverine', 'Estuarine', 'LAcustrine' and 'Coastal' science. The package contains among others: (1) chemical and physical constants and datasets, e.g. atomic weights, gas constants, the earths bathymetry; (2) conversion factors (e.g. gram to mol to liter, barometric units, temperature, salinity); (3) physical functions, e.g. to estimate concentrations of conservative substances, gas transfer and diffusion coefficients, the Coriolis force and gravity; (4) thermophysical properties of the seawater, as from the UNESCO polynomial or from the more recent derivation based on a Gibbs function. License GPL (>= 2) LazyData yes Repository CRAN Repository/R-Forge/Project marelac Repository/R-Forge/Revision 205 Repository/R-Forge/DateTimeStamp 2020-02-11 22:10:45 Date/Publication 2020-02-20 18:50:04 UTC NeedsCompilation yes 1 2 R topics documented: R topics documented: marelac-package . .3 air_density . .5 air_spechum . .6 atmComp . .7 AtomicWeight . .8 Bathymetry . .9 Constants . 10 convert_p . 11 convert_RtoS . 11 convert_salinity . 13 convert_StoCl . 15 convert_StoR . 16 convert_T . 17 coriolis . 18 diffcoeff . 19 earth_surf . 21 gas_O2sat . 23 gas_satconc . 25 gas_schmidt . 27 gas_solubility . 28 gas_transfer . 31 gravity . 33 molvol . 34 molweight . 36 Oceans . 37 redfield . 38 ssd2rad . 39 sw_adtgrad . 40 sw_alpha . 41 sw_beta . 42 sw_comp . 43 sw_conserv . 44 sw_cp . 45 sw_dens . 47 sw_depth . 49 sw_enthalpy . 50 sw_entropy . 51 sw_gibbs . 52 sw_kappa . -

Calculation Exercises with Answers and Solutions
Atmospheric Chemistry and Physics Calculation Exercises Contents Exercise A, chapter 1 - 3 in Jacob …………………………………………………… 2 Exercise B, chapter 4, 6 in Jacob …………………………………………………… 6 Exercise C, chapter 7, 8 in Jacob, OH on aerosols and booklet by Heintzenberg … 11 Exercise D, chapter 9 in Jacob………………………………………………………. 16 Exercise E, chapter 10 in Jacob……………………………………………………… 20 Exercise F, chapter 11 - 13 in Jacob………………………………………………… 24 Answers and solutions …………………………………………………………………. 29 Note that approximately 40% of the written exam deals with calculations. The remainder is about understanding of the theory. Exercises marked with an asterisk (*) are for the most interested students. These exercises are more comprehensive and/or difficult than questions appearing in the written exam. 1 Atmospheric Chemistry and Physics – Exercise A, chap. 1 – 3 Recommended activity before exercise: Try to solve 1:1 – 1:5, 2:1 – 2:2 and 3:1 – 3:2. Summary: Concentration Example Advantage Number density No. molecules/m3, Useful for calculations of reaction kmol/m3 rates in the gas phase Partial pressure Useful measure on the amount of a substance that easily can be converted to mixing ratio Mixing ratio ppmv can mean e.g. Concentration relative to the mole/mole or partial concentration of air molecules. Very pressure/total pressure useful because air is compressible. Ideal gas law: PV = nRT Molar mass: M = m/n Density: ρ = m/V = PM/RT; (from the two equations above) Mixing ratio (vol): Cx = nx/na = Px/Pa ≠ mx/ma Number density: Cvol = nNav/V 26 -1 Avogadro’s -

Lecture 3. the Basic Properties of the Natural Atmosphere 1. Composition
Lecture 3. The basic properties of the natural atmosphere Objectives: 1. Composition of air. 2. Pressure. 3. Temperature. 4. Density. 5. Concentration. Mole. Mixing ratio. 6. Gas laws. 7. Dry air and moist air. Readings: Turco: p.11-27, 38-43, 366-367, 490-492; Brimblecombe: p. 1-5 1. Composition of air. The word atmosphere derives from the Greek atmo (vapor) and spherios (sphere). The Earth’s atmosphere is a mixture of gases that we call air. Air usually contains a number of small particles (atmospheric aerosols), clouds of condensed water, and ice cloud. NOTE : The atmosphere is a thin veil of gases; if our planet were the size of an apple, its atmosphere would be thick as the apple peel. Some 80% of the mass of the atmosphere is within 10 km of the surface of the Earth, which has a diameter of about 12,742 km. The Earth’s atmosphere as a mixture of gases is characterized by pressure, temperature, and density which vary with altitude (will be discussed in Lecture 4). The atmosphere below about 100 km is called Homosphere. This part of the atmosphere consists of uniform mixtures of gases as illustrated in Table 3.1. 1 Table 3.1. The composition of air. Gases Fraction of air Constant gases Nitrogen, N2 78.08% Oxygen, O2 20.95% Argon, Ar 0.93% Neon, Ne 0.0018% Helium, He 0.0005% Krypton, Kr 0.00011% Xenon, Xe 0.000009% Variable gases Water vapor, H2O 4.0% (maximum, in the tropics) 0.00001% (minimum, at the South Pole) Carbon dioxide, CO2 0.0365% (increasing ~0.4% per year) Methane, CH4 ~0.00018% (increases due to agriculture) Hydrogen, H2 ~0.00006% Nitrous oxide, N2O ~0.00003% Carbon monoxide, CO ~0.000009% Ozone, O3 ~0.000001% - 0.0004% Fluorocarbon 12, CF2Cl2 ~0.00000005% Other gases 1% Oxygen 21% Nitrogen 78% 2 • Some gases in Table 3.1 are called constant gases because the ratio of the number of molecules for each gas and the total number of molecules of air do not change substantially from time to time or place to place. -

Interactions Between Polymers and Nanoparticles : Formation of « Supermicellar » Hybrid Aggregates
1 Interactions between Polymers and Nanoparticles : Formation of « Supermicellar » Hybrid Aggregates J.-F. Berret@, K. Yokota* and M. Morvan, Complex Fluids Laboratory, UMR CNRS - Rhodia n°166, Cranbury Research Center Rhodia 259 Prospect Plains Road Cranbury NJ 08512 USA Abstract : When polyelectrolyte-neutral block copolymers are mixed in solutions to oppositely charged species (e.g. surfactant micelles, macromolecules, proteins etc…), there is the formation of stable “supermicellar” aggregates combining both components. The resulting colloidal complexes exhibit a core-shell structure and the mechanism yielding to their formation is electrostatic self-assembly. In this contribution, we report on the structural properties of “supermicellar” aggregates made from yttrium-based inorganic nanoparticles (radius 2 nm) and polyelectrolyte-neutral block copolymers in aqueous solutions. The yttrium hydroxyacetate particles were chosen as a model system for inorganic colloids, and also for their use in industrial applications as precursors for ceramic and opto-electronic materials. The copolymers placed under scrutiny are the water soluble and asymmetric poly(sodium acrylate)-b-poly(acrylamide) diblocks. Using static and dynamical light scattering experiments, we demonstrate the analogy between surfactant micelles and nanoparticles in the complexation phenomenon with oppositely charged polymers. We also determine the sizes and the aggregation numbers of the hybrid organic-inorganic 1 2 complexes. Several additional properties are discussed, such -
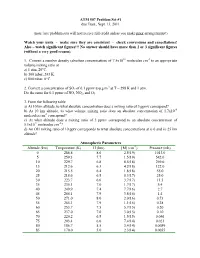
Problem Set #1 Due Tues., Sept
ATM 507 Problem Set #1 due Tues., Sept. 13, 2011 (note: late problem sets will not receive full credit unless you make prior arrangements!) Watch your units - make sure they are consistent - check conversions and cancellations! Also – watch significant figures!!! No answer should have more than 2 or 3 significant figures (without a very good reason). 1. Convert a number density (absolute concentration) of 7.5x1012 molecules cm-3 to an appropriate volume mixing ratio at a) 1 atm, 20°C. b) 100 mbar, 243 K. c) 800 mbar, 0°C. -3 2. Convert a concentration of SO2 of 0.1 ppmv to μg m at T = 298 K and 1 atm. Do the same for 0.1 ppmv of NO, NO2, and O3. 3. From the following table a) At 10 km altitude, to what absolute concentration does a mixing ratio of 3 ppmv correspond? b) At 10 km altitude, to what volume mixing ratio does an absolute concentration of 1.7x1010 molecules cm-3 correspond? c) At what altitude does a mixing ratio of 3 ppmv correspond to an absolute concentration of 5.1x1011 molecules cm-3? d) An OH mixing ratio of 10 pptv corresponds to what absolute concentrations at i) 0 and ii) 25 km altitude? Atmospheric Parameters Altitude (km) Temperature (K) H (km) [M] (cm-3) Pressure (mb) 0 288.8 8.6 2.5(19) 1013.0 5 259.3 7.7 1.5(19) 542.0 10 229.7 6.8 8.5(18) 269.0 15 212.6 6.3 4.2(18) 122.0 20 215.5 6.4 1.8(18) 55.0 25 218.6 6.5 8.3(17) 25.0 30 223.7 6.6 3.7(17) 11.5 35 235.1 7.0 1.7(17) 5.4 40 249.9 7.4 7.7(16) 2.7 45 266.1 7.9 3.8(16) 1.4 50 271.0 8.0 2.0(16) 0.73 55 265.3 7.9 1.1(16) 0.38 60 253.7 7.5 5.7(15) 0.20 65 237.0 7.0 3.0(15) 0.10 70 220.2 6.5 1.5(15) 0.046 75 203.4 6.0 7.4(14) 0.021 80 186.7 5.5 3.4(14) 0.0089 85 170.0 5.0 2.3(14) 0.0055 4. -

Redalyc.Calcite Dissolution by Mixing Waters: Geochemical Modeling And
Geologica Acta: an international earth science journal ISSN: 1695-6133 [email protected] Universitat de Barcelona España SANZ, E.; AYORA, C.; CARRERA, J. Calcite dissolution by mixing waters: geochemical modeling and flow-through experiments Geologica Acta: an international earth science journal, vol. 9, núm. 1, marzo, 2011, pp. 67-77 Universitat de Barcelona Barcelona, España Available in: http://www.redalyc.org/articulo.oa?id=50522124007 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative Geologica Acta, Vol.9, Nº 1, March 2011, 67-77 DOI: 10.1344/105.000001652 Available online at www.geologica-acta.com Calcite dissolution by mixing waters: geochemical modeling and flow-through experiments 1 2 1 E. SANZ C. AYORA J. CARRERA 1 ExxonMobil Upstream Research Company Houston, Texas, USA E-mail: [email protected] 2 Institute of Enviromental Assessment and Water Research, IDAEA-ECSIC Barcelona, Spain ABSTRACT TABLE CAPTIONS Dissolution of carbonates has been commonly predicted by geochemical models to occur at the seawater-freshwater mixing zone of coastal aquifers along a geological time scale. However, field evidences are inconclusive:dissolution TABLE 1 Input end-member solutions used in the experiments and numerical simulations. Ω accounts for saturation, and I for ionic strength. vs. lack of dissolution. In this study we investigate the process of calcite dissolution by mixing waters of different salinities and pCO2, by means of geochemical modeling and laboratory experiments. -

Mole Fraction - Wikipedia
4/28/2020 Mole fraction - Wikipedia Mole fraction In chemistry, the mole fraction or molar fraction (xi) is defined as unit of the amount of a constituent (expressed in moles), ni divided by the total amount of all constituents in a mixture (also [1] expressed in moles), ntot: .These expression is given below:- The sum of all the mole fractions is equal to 1: The same concept expressed with a denominator of 100 is the mole percent or molar percentage or molar proportion (mol%). The mole fraction is also called the amount fraction.[1] It is identical to the number fraction, which is defined as the number of molecules of a constituent Ni divided by the total number of all molecules Ntot. The mole fraction is sometimes denoted by the lowercase Greek letter χ (chi) instead of a Roman x.[2][3] For mixtures of gases, IUPAC recommends the letter y.[1] The National Institute of Standards and Technology of the United States prefers the term amount-of- substance fraction over mole fraction because it does not contain the name of the unit mole.[4] Whereas mole fraction is a ratio of moles to moles, molar concentration is a quotient of moles to volume. The mole fraction is one way of expressing the composition of a mixture with a dimensionless quantity; mass fraction (percentage by weight, wt%) and volume fraction (percentage by volume, vol%) are others. Contents Properties Related quantities Mass fraction Molar mixing ratio Mixing binary mixtures with a common component to form ternary mixtures Mole percentage Mass concentration Molar concentration Mass and molar mass Spatial variation and gradient References https://en.wikipedia.org/wiki/Mole_fraction 1/5 4/28/2020 Mole fraction - Wikipedia Properties Mole fraction is used very frequently in the construction of phase diagrams. -

1 Atmospheric Sciences 501. Course Notes Autumn 1998. 1. Ideal Gas
1 Atmospheric Sciences 501. Course Notes Autumn 1998. 1. Ideal Gas Law and Hydrostatics. 1.1 Ideal Gas Law. Basic thermodynamic variables are: Pressure, p (N m-2; 1 N m-2 = 1 pascal (Pa) = 0.01 millibar (mb) = 0.01hectoPascals (hPa). Density, r (kgm-3) Temperature, T (K) . If the energy due to intermolecular forces is small relative to molecular kinetic energy, the ideal gas law is an excellent approximation to the equation of state relating these variables: pRT=r (1.1) R* where R = = gas constant; R* = universal gas constant = 8314J kmol-1 K-1; M = molar M weight (kg/kg-mole) and R = specific gas constant for dry air = 287 J kg-1 K-1. 1.2 Molecules and the ideal gas law, Maxwell's kinetic interpretation. Note that r=nmÃà (1.2) where nà = number density (number of molecules per unit volume m-3), and mà = molecular mass (kg). Also MmA= à (1.3) where A = Avogadro's Number = 6.022x1026 molecules per mole; note that, since SI units are used, moles here are kilogram-moles, not gram-moles. Substituting (1.2) and (1.3) into (1.1): 2 æ R* ö p = ç ÷ nTÃÃ= knT (1.4) è A ø * æ R ö -23 -1 where k = ç ÷ = 1.381x10 J K is Boltzmann's Constant. è A ø Now consider the pressure exerted by molecules of gas on a surface perpendicular to the x- axis in a Cartesian reference frame. This is the force per unit area and is equivalent to the average flux in the x-direction of molecules with x-momentum mvà , mvÃà nv x ()xx× where () represents averaging over molecular velocities. -

The Atmosphere
The Atmosphere Atmospheric composition Measures of concentration Atmospheric pressure Barometric law Literature connected with today’s lecture: Jacob, chapter 1 - 2 Exercises: 1:1 – 1:6; 2:1 – 2:4 The Atmosphere The atmosphere –Thin “skin” of air surrounding the planet Description Role of natural atmosphere Effects of changed composition Atmospheric change Climate UV protection Acidification Health 1 Composition of the Atmosphere Dry atmosphere (excl. H2O): Gas Mixing ratio Dry atmosphere: (mole/mole) Dominated by nitrogen and oxygen Nitrogen (N2)0.78 Noble gases, in particular argon Oxygen (O2)0.21 Argon (Ar) 0.0093 Conc. (O2 + N2 + Ar) 1 mole/mole -6 Remaining components trace gases: E.g. Carbon dioxide (CO2) 365x10 Carbon dioxide, ozone, methane Neon (Ne) 18x10-6 -6 Ozone (O3) 0.01-10x10 Helium (He) 5.2x10-6 Humid atmosphere: -6 Water vapour: Varies, up to approx. 0.03 Methane (CH4) 1.7x10 moles/mole Krypton (Kr) 1.1x10-6 -9 Hydrogen (H2) 500x10 -9 Nitrous oxide (N2O) 320x10 Calculation Example: Calculate the density of dry air at T = 280 K and P = 1000 hPa! The atmosphere an ideal gas (in most cases): PV = nRT Density: = m/V = nM/V From the gas law: n/V = P/RT => =MP/RT Air is a mixture of gases – average molar mass: Ma = 29,0 kg/kmole Gas constant: R = 8314,3 J/(kmole K) Insert numbers: 3 = MaP/RT = 29,0*1000*100/(280*8314,3) = 1,25 kg/m 2 Atmospheric Concentration of Species Mixing ratio: Expressions atmospheric concentration: (No. moles of X)/(No. moles air molecules) Number concentration: Or: mass X/mass -

Measurements of the HONO Photodissociation Constant
Measurements ofthe HONO photodissociation constant K. J. Wall· C. L. SchilIer· G. W. Harris Abstract Measurements of the photodissociation constant for nitrous acid (jHONO) were made at an urban site in Toronto, Canada, during the months of May-July 2005, using an optically thin actinometer. Operating details of the jHONO monitor are reported, along with laboratory tests. Measurements of jHONO were obtained for solar zenith angles ranging 0 from 20-75 , under clear and cloudy skies. Maximum error estimates on jHONO under clear skies range from 11 % at sunrise, to 4% at solar noon, with a minimum detection limit of 4 5.7 x 10- /sec for our actinometer. Measured clear-sky values of jHONO were compared with values calculated by a four-stream discrete ordinate radiative transfer (RT) model (ACD TUV version 4.1), and were found to be within better than 10% agreement for solar zenith angles 0 <65 • For conditions of scattered cloud, enhancement and suppression of the jHONO values occurred by as much as 16%-70%, and 59%-80%, respectively. The integrated band area of the mr* transition for gas-phase nitrous acid yields an oscillator strength, f = (1.06 ± 0.044) x 10-3 (based on clear-sky data), 19.1 % higher than the value reported by Bongartz et al. (1991). Keywords Actinic flux· Actinometer· Nitrous acid (HONO) . Radiative transfer model 1. Introduction It has long been recognized that the photolysis of nitrous acid (HONO) is a potentially important production mechanism for hydroxyl radicals in the polluted urban atmosphere (Harris et al., 1982). In the wavelength range of 300-400 nm, photolysis of HONO directly produces OH: HONO+hv ~ OH+NO (Ri) The hydroxyl radical is central in the formation ofozone and is the most important oxidizing species in the daytime atmosphere.