Hip Resurfacing
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Patient Guide for a Hip Replacement
Day of surgery: ____________________ Expected discharge home: ____________________ Surgeon: ____________________ Phone number: ____________________ Bring this guide with you to the hospital on the day of your surgery 4100492 (17-10) 2 Introduction This information guide will help you understand what is involved in hip arthroplasty (hip replacement). We hope the information in this booklet will help you prepare for your surgery •••••••••••••••••••••••••••••••••••••••••••••••••••••• About Hôpital Montfort Hôpital Montfort is a Francophone academic health care institution that provides quality care in both official languages and works with its partners to improve the health of communities. Montfort strives for excellence and wants to be a hospital of choice for its personalized patient care, its workplace, teaching and research. Our daily actions are guided by the values of compassion, commitment, excellence and respect. WARNING This guide does not replace the advice of your care provider. Please consult your care provider to assess if the information presented in this guide applies to your situation. The content of this guide was prepared by Vancouver Coastal Health and adapted by Hôpital Montfort. 3 Your Health Care: Be Involved! • Be involved in your health care. Speak up if you have questions or concerns about your care. • Tell a member of your health care team about your past illnesses and your current health condition. • Tell your health team if you have any food or medication allergies. • Make sure you know what to do when you leave the hospital. •••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••• Your Interprofessional Plan of Care • Your hospital admission for your total hip replacement will follow an “interprofessional plan of care” more commonly called a "Clinical Pathway". -
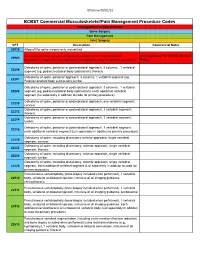
Commercial MSK Procedure Code List Effective 09.01.21.Xlsx
Effective 09/01/21 BCBST Commercial Musculoskeletal/Pain Management Procedure Codes Investigational or Non-Covered Spine Surgery Pain Management Joint Surgery CPT Description Commercial Notes 20930 Allograft for spine surgery only morselized Computer-assisted surgical navigational procedure for musculoskeletal Investigational Per BCBST Medical 20985 procedures, image-less (List separately in addition to code for primary procedure) Policy Osteotomy of spine, posterior or posterolateral approach, 3 columns, 1 vertebral 22206 segment (eg, pedicle/vertebral body subtraction); thoracic Osteotomy of spine, posterior approach, 3 columns, 1 vertebral segment (eg. 22207 Pedicle/vertebral body subtraction);lumbar Osteotomy of spine, posterior or posterolateral approach, 3 columns, 1 vertebral 22208 segment (eg, pedicle/vertebral body subtraction); each additional vertebral segment (list separately in addition to code for primary procedure) Osteotomy of spine, posterior or posterolateral approach, one vertebral segment; 22210 cervical Osteotomy of spine, posterior or posterolateral approach, 1 vertebral segment; 22212 thoracic Osteotomy of spine, posterior or posterolateral approach, 1 vertebral segment; 22214 lumbar Osteotomy of spine, posterior or posterolateral approach, 1 vertebral segment; 22216 each additional vertebral segment (List separately in addition to primary procedure) Osteotomy of spine, including discectomy anterior approach, single vertebral 22220 segment; cervical Osteotomy of spine, including discectomy, anterior approach, single -

Hip Impingement and Osteoarthritis. Hip Arthroscopy, Resurfacing, Or Replacement: Is It Time for Surgery?
Hip Impingement and Osteoarthritis. Hip Arthroscopy, Resurfacing, or Replacement: Is it Time for Surgery? Bryan Whitfield, MD [email protected] Hip Operations • Background of hip diagnoses • Treatment of hip pathology • Surgery candidates • Time for surgery? • Cases Intra-articular hip pain - diagnoses • FAI (femoroacetabular impingement) / acetabular labrum tear • Neck of the femur and rim of the socket run into each other • Tear the labrum. • Articular cartilage damage possible • Labrum: fluid management, stability, and seal of the joint • Osteoarthritis • Socket and ball both coated in a layer of cartilage • Arthritis is the wearing away, loss of or damage to the cartilage coating (articular cartilage) Intra-articular hip pain - diagnoses • FAI (femoroacetabular impingement) / acetabular labrum tear • Neck of the femur and rim of the socket run into each other • Tear the labrum. • Articular cartilage damage possible • Labrum: fluid management, stability, and seal of the joint • Osteoarthritis • Socket and ball both coated in a layer of cartilage • Arthritis is the wearing away, loss of or damage to the cartilage coating (articular cartilage) Intra-articular hip pain - diagnoses • FAI (femoroacetabular impingement) / acetabular labrum tear • Neck of the femur and rim of the socket run into each other • Tear the labrum. • Articular cartilage damage possible • Labrum: fluid management, stability, and seal of the joint • Osteoarthritis • Socket and ball both coated in a layer of cartilage • Arthritis is the wearing away, loss -

Dr. Mark Dolan Specializes in Hip and Knee Replacement Surgery, Hip Resurfacing, and Hip Arthroscopy/Hip Preservation Surgery
Dr. Mark Dolan specializes in hip and knee replacement surgery, hip resurfacing, and hip arthroscopy/hip preservation surgery. Dr. Dolan is originally from South Bend, Indiana and a graduate of the University of Notre Dame. He attended Indiana University School of Medicine before moving to Chicago to continue his education in orthopaedic surgery as a resident in Northwestern University Feinberg School of Medicine’s Department of Orthopaedic Surgery. After residency he completed a fellowship in Adult Reconstruction and Joint Replacement Surgery at the Hospital for Special Surgery in New York where he gained advanced training in hip and knee replacement surgery, hip resurfacing, and revision total joint surgery. Following this year long fellowship, he stayed at the Hospital for Special Surgery to obtain additional experience in providing care for young adults with hip pain. This included additional study in hip arthroscopy and hip preservation surgery. Dr. Dolan has a specific interest in hip disorders and strives to provide comprehensive care to patients suffering from hip pain. This includes non-operative treatment as well as total joint replacement, hip resurfacing, and arthroscopic hip surgery (athletic hip injuries, hip joint preservation surgery, femoracetabular impingement (FAI), and acetabular labral injuries). Dr. Dolan utilizes this wide array of treatment options to best serve his patients, working with each patient to develop a focused and individualized treatment plan. As a former varsity soccer player at Notre Dame and an active runner and skier, Dr. Dolan appreciates the importance of maintaining an active lifestyle. His goal is to relieve your pain and improve your mobility, thereby allowing you to return to an active life. -

PART G Lower Extremity Treatment Guidelines.Fm
TITLE 19 LABOR 1 DELAWARE ADMINISTRATIVE CODE 1000 DEPARTMENT OF LABOR 1300 Division of Industrial Affairs 1340 The Office of Workers’ Compensation 1342 Health Care Practice Guidelines PART G Lower Extremity Treatment Guidelines 1.0 Introduction Pursuant to 19 Del.C. §2322C, health care practice guidelines have been adopted and recommended by the Health Care Advisory Panel to guide utilization of health care treatments in workers' compensation including, but not limited to, care provided for the treatment of employees by or under the supervision of a licensed health care provider, prescription drug utilization, inpatient hospitalization and length of stay, diagnostic testing, physical therapy, chiropractic care and palliative care. The health care practice guidelines apply to all treatments provided after the effective date of the regulation adopted by the Department of Labor, May 23, 2008, and regardless of the date of injury. Lower Extremity was added to the list of treatment guidelines, effective 6/13/2011. The guidelines are, to the extent permitted by the most current medical science or applicable science, based on well-documented scientific research concerning efficacious treatment for injuries and occupational disease. To the extent that well-documented scientific research regarding the above is not available at the time of adoption of the guidelines, or is not available at the time of any revision to the guidelines, the guidelines have been and will be based upon the best available information concerning national consensus regarding best health care practices in the relevant health care community. The guidelines, to the extent practical and consistent with the Act, address treatment of those physical conditions which occur with the greatest frequency, or which require the most expensive treatments, for work-related injuries based upon currently available Delaware data. -

IC5-Fixing-The-Femurs-Fibres-And
27 LOWER LIMB FUNCTION Pam Thomason, Jill Rodda, Kate Willoughby, and H Kerr Graham Introduction anteversion (FNA) increases from GMFCS level I to level III The 2006 definition of cerebral palsy (CP) has been widely and then plateaus at a mean of 40 degrees in GMFCS levels accepted and is recommended as the most useful operational III, IV and V. Mean neck shaft angle, increases stepwise from definition of CP (Rosenbaum et al. 2007). This definition GMFCS level I through to GMFCS level V (Robin et al. 2008). includes the term ‘secondary musculoskeletal problems’ as The incidence and severity of hip displacement is directly pre- part of the spectrum of features seen in CP. This recognizes dicted by GMFCS level. In our population-based study, chil- the impact of musculoskeletal pathologies on functioning dren in GMFCS level I showed no hip displacement and those for persons with CP. The focus of this section will be the in GMFCS level V had a 90% incidence of having a migration effects of the brain lesion on the motor function of the lower percentage in excess of 30% (Soo et al. 2006). The relationship limbs, pelvis, and spine. The authors work together in a gait between GMFCS and hip displacement has implications for laboratory and hip surveillance clinic with a commitment screening and management protocols. to provide clinical services and to improve the evidence base Knowing a child’s GMFCS level is fundamental in by clinical research. The framework for this section will be establishing gross motor prognosis and monitoring changes based on the World Health Organization International (Rosenbaum et al. -

Adverse Reactions to Metal Debris After Fully-Modular Primary Total Hip Arthroplasty: a Report of Two Cases
Central JSM Bone and Joint Diseases Bringing Excellence in Open Access Case Report *Corresponding author Suguru Ohsawa, Department of Physical Therapy, Osaka Yukioka College of Health Science, 2-2-3 Ukita, Adverse Reactions to Metal Kita-Ku, Osaka 530-0021, Japan, Tel: 81-6-6371-9921, FAX: 81-6-6371-6778; Email: Debris after Fully-Modular Submitted: 23 February 2017 Accepted: 18 March 2017 Primary Total Hip Arthroplasty: Published: 20 March 2017 Copyright A Report of Two Cases © 2017 Hira et al. OPEN ACCESS Kayo Hira1, Makoto Iwasa1, Keisuke Akiyama1, Takaaki Shibuya1, Shigeki Fujita2, and Suguru Ohsawa3* Keywords • Metal debris 1Department of Orthopaedic Surgery, Sumitomo Hospital • Neck-head junctions 2Department of Pathology, Sumitomo Hospital • Arthroplasty 3Department of Physical Therapy, Osaka Yukioka College of Health Science Abstract Adverse reactions to metal debris (ARMDs) are a current concern in total hip arthroplasty (THA). The occurrence of ARMDs is well documented in metal-on-metal (MoM) resurfacing and THA and corrosion at a modular neck-body junction with a metal-on-polyethylene (MoP) bearing THA has recently been reported. However, dual- modular primary THA including modular stem-neck and neck-head junctions is not well documented. We performed 18 MoP THAs with a 40-mm diameter head (2011-2016), and two patients required revision arthroplasty for a symptomatic pseudotumor with corrosion, at the modular head-neck junction in one and at the neck-body junction in the other. INTRODUCTION the institutional ethics committee and was therefore performed in accordance with the ethical standards laid down in the latest Adverse reactions to metal debris (ARMDs) are a current version of the 1964 Declaration of Helsinki. -

Evaluation and Treatment of the Painful Hip Resurfacing
9 Review Article Page 1 of 9 Evaluation and treatment of the painful hip resurfacing Edwin P. Su1,2 1Department of Orthopedic Surgery, Weill Cornell Medical College, New York, NY, USA; 2Adult Reconstruction and Joint Replacement Division, Hospital for Special Surgery, New York, NY, USA Correspondence to: Edwin P. Su, MD. 535 East 70th Street, New York, NY 10021, USA. Email: [email protected]. Abstract: Hip resurfacing arthroplasty (HRA) can have unique failure mechanisms not commonly seen with traditional metal on polyethylene total hip replacements (THRs) due to the shape and fixation of the implants, interaction of the bone with the implants, biomechanics of the joint, and the metal-on-metal (MOM) articulation. As such, one should be aware of the most common mechanisms for pain in a hip resurfacing to be able to evaluate the problem with the proper imaging and laboratory studies. Besides the usual causes of pain in a hip arthroplasty such as infection, hip flexor tendonitis, and implant loosening, there can also be bone-to-implant impingement, synovitis from metal debris, and immunologic reaction to metal debris. Thus, the diagnosis of problems in a metal on metal hip resurfacing is aided by the use of diagnostic modalities such as metal ion measurement and metal artifact reduction imaging techniques. The treatment of such problems can consist of a revision to a stemmed THR, either by revising the femoral component only, or performing a revision of both components. Keywords: Hip resurfacing; revision; metal-on-metal (MOM); painful hip Received: 10 September 2019; Accepted: 02 December 2019; Published: 15 April 2020. -

Hip Resurfacing (Re-Review)
20, 2012 Health Technology Assessment Hip Resurfacing (Re-Review) Final Evidence Report October 14, 2013 Health Technology Assessment Program (HTA) Washington State Health Care Authority PO Box 42712 Olympia, WA 98504-2712 (360) 725-5126 hta.hca.wa.gov [email protected] Hip Resurfacing (Re-Review) Provided by: Spectrum Research, Inc. Prepared by: Joseph R. Dettori, PhD, MPH Robin Hashimoto, PhD Katie Moran, BS Erika Ecker, BS October 14, 2013 This technology assessment report is based on research conducted by a contracted technology assessment center, with updates as contracted by the Washington State Health Care Authority. This report is an independent assessment of the technology question(s) described based on accepted methodological principles. The findings and conclusions contained herein are those of the investigators and authors who are responsible for the content. These findings and conclusions may not necessarily represent the views of the HCA/Agency and thus, no statement in this report shall be construed as an official position or policy of the HCA/Agency. The information in this assessment is intended to assist health care decision makers, clinicians, patients and policy makers in making sound evidence-based decisions that may improve the quality and cost- effectiveness of health care services. Information in this report is not a substitute for sound clinical judgment. Those making decisions regarding the provision of health care services should consider this report in a manner similar to any other medical reference, integrating the information with all other pertinent information to make decisions within the context of individual patient circumstances and resource availability. WA - Health Technology Assessment October 14, 2013 TABLE OF CONTENTS EXECUTIVE SUMMARY .......................................................................................................................1 1. -

The Durability of Hip Resurfacing
THE DURABILITY OF HIP RESURFACING Thomas P. Gross, MD 13 years experience. Over 3000 cases. For more information: grossortho.com A recent Article in the Lancet medical journal has criticized hip resurfacing arthroplasty (HRA) as less durable than cemented 28mm total hip replacement (THR). I take exception to the inappropriate conclusion that the authors drew from this highly flawed study. However, there are two conclusions that can be drawn from this study. Surgeons, who are inexperienced in hip resurfacing, have more revisions in the short term with resurfacing than if they stick with standard hip replacement. Women have a higher failure rate than men with hip resurfacing. Both of these are old news. Hip resurfacing has several distinct advantages over stemmed small bearing total hip replacement. The resurfaced hip most closely resembles the natural hip. Biomechanically the hip is stable, most of the bone is preserved and stresses are transferred more naturally to the remaining bone. Near normal function can be achieved, even by impact athletes. Thigh pain (3-5% of stemmed THR) does not occur in resurfacing. Dislocation is rare (<0.5%), and revision for recurrent instability is extremely rare (0.03%). The problem is not with resurfacing. The first problem is that two poorly designed hip resurfacing implant systems were released into the marketplace. The second problem is that too many surgeons have dabbled in resurfacing and never achieved enough experience with this difficult operation to get over the learning curve. It is worth emphasizing that the average surgeon volume for resurfacing in the Lancet study was 3.7 cases / year. -

Safety-Measures-Hip-Arthroscopy.Pdf
Systematic Review Safety Measures in Hip Arthroscopy and Their Efficacy in Minimizing Complications: A Systematic Review of the Evidence Asheesh Gupta, M.D., John M. Redmond, M.D., Jon E. Hammarstedt, B.S., Leslie Schwindel, M.D., and Benjamin G. Domb, M.D. Purpose: The purpose of this systematic review was to evaluate the literature to determine complications of hip arthroscopy, with a secondary focus on how to minimize complications and risks. Methods: Two independent reviewers performed a search of PubMed for articles that contained at least 1 of the following terms: complications and hip arthroscopy, hip impingement, femoral acetabular impingement and complications, or femoroacetabular impingement (FAI) and complications. The search was limited to articles published between 1999 and June 2013. An additional search was performed for articles evaluating techniques on how to minimize complications. Results: We identified 81 studies (5,535 patients; 6,277 hips). The mean age was 35.48 years, and the mean body mass index was 25.20 kg/m2. Of the participants, 52% were male and 48% were female. The majority of studies were Level IV Evidence (63%). A total of 285 complications were reported, for an overall rate of 4.5%. There were 26 major complications (0.41%) and a 4.1% minor complication rate. The overall reoperation rate was 4.03%. A total of 94 hips underwent revision arthroscopy. Regarding open procedures, 150 patients (93%) underwent either total hip arthroplasty or a hip resurfacing procedure. The conversion rate to total hip arthroplasty or a resurfacing procedure was 2.4%. Conclusions: Overall, primary hip arthroscopy is a successful procedure with low rates of major (0.41%) and minor (4.1%) complications. -

Evidence-Based Clinical Practice Guideline
Detection and Nonoperative Management of Pediatric Developmental Dysplasia of the Hip in Infants up to Six Months of Age Evidence-Based Clinical Practice Guideline Adopted by: The American Academy of Orthopaedic Surgeons Board of Directors September 5, 2014 Endorsed by: Please cite this Clinical Practice Guideline as: American Academy of Orthopaedic Surgeons Evidence- Based Clinical Practice Guideline for the Detection and Nonoperative Management of Pediatric Dysplasia of the Hip in Infants Up to Six Months of Age. https://www.aaos.org/globalassets/quality-and-practice- resources/pddh/pediatric-developmental-dysplasia-hip-clinical-practice-guideline-4-23-19.pdf Published September 4, 2014 Disclaimer This Clinical Practice Guideline was developed by an AAOS clinician volunteer Work Group based on a systematic review of the current scientific and clinical information and accepted approaches to treatment and/or diagnosis. This Clinical Practice Guideline is not intended to be a fixed protocol, as some patients may require more or less treatment or different means of diagnosis. Clinical patients may not necessarily be the same as those found in a clinical trial. Patient care and treatment should always be based on a clinician’s independent medical judgment, given the individual patient’s clinical circumstances. Disclosure Requirement In accordance with AAOS policy, all individuals whose names appear as authors or contributors to Clinical Practice Guideline filed a disclosure statement as part of the submission process. All panel members provided full disclosure of potential conflicts of interest prior to voting on the recommendations contained within this Clinical Practice Guidelines. Funding Source This Clinical Practice Guideline was funded exclusively by the American Academy of Orthopaedic Surgeons who received no funding from outside commercial sources to support the development of this document.