Elasto-Optic, Electro-Optic, and Magneto-Optic Constants
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Standard X-Ray Diffraction Powder Patterns
NBS MONOGRAPH 25 — SECTION 1 Standard X-ray Diffraction U.S. DEPARTMENT OF COMMERCE NATIONAL BUREAU OF STANDARDS THE NATIONAL BUREAU OF STANDARDS Functions and Activities The functions of the National Bureau of Standards are set forth in the Act of Congress, March 3, 1901, as amended by Congress in Public Law 619, 1950. These include the development and maintenance of the national standards of measurement and the provision of means and methods for making measurements consistent with these standards; the determination of physical constants and properties of materials; the development of methods and instruments for testing materials, devices, and structures; advisory services to government agencies on scien- tific and technical problems; invention and development of devices to serve special needs of the Government; and the development of standard practices, codes, and specifications. The work includes basic and applied research, development, engineering, instrumentation, testing, evaluation, calibration services, and various consultation and information services. Research projects are also performed for other government agencies when the work relates to and supplements the basic program of the Bureau or when the Bureau's unique competence is required. The scope of activities is suggested by the listing of divisions and sections on the inside of the back cover. Publications The results of the Bureau's research are published either in the Bureau's own series of publications or in the journals of professional and scientific societies. The Bureau itself publishes three periodicals available from the Government Printing Office: The Journal of Research, published in four separate sections, presents complete scientific and technical papers; the Technical News Bulletin presents summary and preliminary reports on work in progress; and Basic Radio Propagation Predictions provides data for determining the best frequencies to use for radio communications throughout the world. -

In-Depth Analysis by Co-Imaging of Periodically-Poled X-Cut Lithium Niobate Thin Films
crystals Article “Seeing Is Believing”—In-Depth Analysis by Co-Imaging of Periodically-Poled X-Cut Lithium Niobate Thin Films Sven Reitzig 1,* , Michael Rüsing 1, Jie Zhao 2,†, Benjamin Kirbus 1, Shayan Mookherjea 2 and Lukas M. Eng 1,3 1 Institut für Angewandte Physik, Technische Universität Dresden, 01062 Dresden, Germany; [email protected] (M.R.); [email protected] (B.K.); [email protected] (L.M.E.) 2 Department of Electrical and Computer Engineering, University of California, San Diego, CA 92161, USA; [email protected] (J.Z.); [email protected] (S.M.) 3 ct.qmat: Dresden-Würzburg Cluster of Excellence—EXC 2147, TU Dresden, 01062 Dresden, Germany * Correspondence: [email protected]; Tel.: +49-351-463-43354 † Since February 2021 affiliated with Nokia Bell Labs, Murray Hill, NJ 07974, USA. Abstract: Nonlinear and quantum optical devices based on periodically-poled thin film lithium niobate (PP-TFLN) have gained considerable interest lately, due to their significantly improved performance as compared to their bulk counterparts. Nevertheless, performance parameters such as conversion efficiency, minimum pump power, and spectral bandwidth strongly depend on the quality of the domain structure in these PP-TFLN samples, e.g., their homogeneity and duty cycle, as well as on the overlap and penetration depth of domains with the waveguide mode. Hence, in order to propose improved fabrication protocols, a profound quality control of domain structures is needed that allows quantifying and thoroughly analyzing these parameters. In this paper, we Citation: Reitzig, S.; Rüsing, M.; propose to combine a set of nanometer-to-micrometer-scale imaging techniques, i.e., piezoresponse Zhao, J.; Kirbus, B.; Mookherjea, S.; force microscopy (PFM), second-harmonic generation (SHG), and Raman spectroscopy (RS), to access Eng, L.M. -

High Temperature Ultrasonic Transducers: a Review
sensors Review High Temperature Ultrasonic Transducers: A Review Rymantas Kazys * and Vaida Vaskeliene Ultrasound Research Institute, Kaunas University of Technology, Barsausko st. 59, LT-51368 Kaunas, Lithuania; [email protected] * Correspondence: [email protected] Abstract: There are many fields such as online monitoring of manufacturing processes, non-destructive testing in nuclear plants, or corrosion rate monitoring techniques of steel pipes in which measure- ments must be performed at elevated temperatures. For that high temperature ultrasonic transducers are necessary. In the presented paper, a literature review on the main types of such transducers, piezoelectric materials, backings, and the bonding techniques of transducers elements suitable for high temperatures, is presented. In this review, the main focus is on ultrasonic transducers with piezoelectric elements suitable for operation at temperatures higher than of the most commercially available transducers, i.e., 150 ◦C. The main types of the ultrasonic transducers that are discussed are the transducers with thin protectors, which may serve as matching layers, transducers with high temperature delay lines, wedges, and waveguide type transducers. The piezoelectric materi- als suitable for high temperature applications such as aluminum nitride, lithium niobate, gallium orthophosphate, bismuth titanate, oxyborate crystals, lead metaniobate, and other piezoceramics are analyzed. Bonding techniques used for joining of the transducer elements such as joining with glue, soldering, -
![Arxiv:2002.06594V2 [Physics.Optics] 26 Oct 2020 Tives to the Metallic Nanostructures](https://docslib.b-cdn.net/cover/9256/arxiv-2002-06594v2-physics-optics-26-oct-2020-tives-to-the-metallic-nanostructures-269256.webp)
Arxiv:2002.06594V2 [Physics.Optics] 26 Oct 2020 Tives to the Metallic Nanostructures
Resonantly tunable second harmonic generation from lithium niobate metasurfaces Junjun Ma,1 Fei Xie,1 Weijin Chen,2 Jiaxin Chen,1 Wei Wu,1 Wei Liu,3 Yuntian Chen,2 Wei Cai,1 Mengxin Ren,1, 4, a) and Jingjun Xu1, b) 1)The Key Laboratory of Weak-Light Nonlinear Photonics, Ministry of Education, School of Physics and TEDA Applied Physics Institute, Nankai University, Tianjin 300071, P.R. China 2)School of Optical and Electronic Information, Huazhong University of Science and Technology, Wuhan, Hubei 430074, P.R.China 3)College for Advanced Interdisciplinary Studies, National University of Defense Technology, Changsha, Hunan 410073, P.R.China 4)Collaborative Innovation Center of Extreme Optics, Shanxi University, Taiyuan, Shanxi 030006, P.R. China (Dated: 27 October 2020) Second harmonic generation (SHG) is a coherent nonlinear phenomenon that plays an important role in laser color conversion. Lithium niobate (LN), which features both a large band gap and outstanding second-order nonlinearities, acts as an important optical material for nonlinear frequency conversion covering a wide spectral range from ultraviolet to mid-infrared. Here we experimentally demonstrate LN metasurfaces with controllable SHG properties. Distinct enhancements for the SHG efficiency are observed at Mie-resonances. And by changing the geometric parameters thus the resonances of the metasurfaces, we manage to selectively boost the SHG efficiency at different wavelengths. Our results would pave a way for developing with high flexibility the novel compact nonlinear light sources for applications, such as biosensing and optical communications. Keywords: Lithium niobate, Metasurface, Second harmonic generation, Resonance Second harmonic generation (SHG) plays a key role diffraction limit. -

Optical Properties of KTP Crystals and Their Potential for Terahertz Generation
crystals Article Optical Properties of KTP Crystals and Their Potential for Terahertz Generation Alexander Mamrashev 1,* ID , Nazar Nikolaev 1 ID , Valery Antsygin 1, Yury Andreev 2,3, Grigory Lanskii 2,3 and Arkady Meshalkin 4 1 Institute of Automation & Electrometry, SB RAS, 1, Ac. Koptyug Ave., Novosibirsk 630090, Russia; [email protected] (N.N.); [email protected] (V.A.) 2 Institute of Monitoring of Climatic and Ecological Systems, SB RAS, 10/3, Akademicheskii Ave., Tomsk 634055, Russia; [email protected] (Y.A.); [email protected] (G.L.) 3 Siberian Physical Technical Institute of Tomsk State University, 1 Novosobornaya Sq., Tomsk 634050, Russia 4 Institute of Thermophysics, SB RAS, 1, Ac. Lavrent’ev Ave., Novosibirsk 630090, Russia; [email protected] * Correspondence: [email protected]; Tel.: +7-383-330-8378 Received: 30 June 2018; Accepted: 28 July 2018; Published: 31 July 2018 Abstract: High nonlinearity, wide transparency range and optical quality allowed potassium titanyl phosphate (KTiOPO4, KTP) crystals to be used in a wide range of nonlinear applications. The success of KTP usage in the visible and infrared (IR) ranges drives interest in applying it at longer wavelengths, that is, in the terahertz (THz) range. We use THz optical properties of KTP crystals measured by terahertz time-domain spectrometer (THz-TDS) and refractive index approximated in the form of Sellmeier equations to investigate KTP application possibilities for IR-to-THz and THz-to-THz wave conversion. As a result, phase matching for s − f ! f and s − f ! s types of difference frequency generation (DFG) of Ti:Sapphire laser (at the wavelengths of 0.65, 0.8 and 1.1 µm) is found possible, as well as second harmonic generation (SHG) of THz waves by f + s ! f type of interaction in the XZ principle plane of the crystal. -

Integrated Photonics on Thin-Film Lithium Niobate
Integrated photonics on thin-film lithium niobate DI ZHU,1,* LINBO SHAO,1 MENGJIE YU,1 REBECCA CHENG,1 BORIS DESIATOV,1 C. J. XIN,1 YAOWEN HU,1 JEFFREY HOLZGRAFE,1 SOUMYA GHOSH,1 AMIRHASSAN SHAMS-ANSARI,1 ERIC PUMA,1 NEIL SINCLAIR,1,2 CHRISTIAN REIMER,3 MIAN ZHANG,3 AND MARKO LONČAR1,* 1John A. Paulson School of Engineering and Applied Sciences, Harvard University, 29 Oxford Street, Cambridge, Massachusetts 02138, USA 2Division of Physics, Mathematics and Astronomy, and Alliance for Quantum Technologies (AQT), California Institute of Technology, 1200 E. California Boulevard, Pasadena, CA 91125, USA 3HyperLight Corporation, 501 Massachusetts Avenue, Cambridge, Massachusetts 02139, USA [email protected], [email protected] Lithium niobate (LN), an outstanding and versatile material, has influenced our daily life for decades: from enabling high-speed optical communications that form the backbone of the Internet to realizing radio-frequency filtering used in our cell phones. This half-century-old material is currently embracing a revolution in thin-film LN integrated photonics. The success of manufacturing wafer-scale, high-quality, thin films of LN on insulator (LNOI), accompanied with breakthroughs in nanofabrication techniques, have made high-performance integrated nanophotonic components possible. With rapid development in the past few years, some of these thin-film LN devices, such as optical modulators and nonlinear wavelength converters, have already outperformed their legacy counterparts realized in bulk LN crystals. Furthermore, the nanophotonic integration enabled ultra-low-loss resonators in LN, which unlocked many novel applications such as optical frequency combs and quantum transducers. In this Review, we cover—from basic principles to the state of the art—the diverse aspects of integrated thin- film LN photonics, including the materials, basic passive components, and various active devices based on electro-optics, all-optical nonlinearities, and acousto-optics. -

A Study of Lithium Precursors on Nanoparticle Quality
Electronic Supplementary Material (ESI) for Nanoscale. This journal is © The Royal Society of Chemistry 2021 Electronic Supplementary Information Elucidating the role of precursors in synthesizing single crystalline lithium niobate nanomaterials: A study of lithium precursors on nanoparticle quality Rana Faryad Ali, Byron D. Gates* Department of Chemistry and 4D LABS, Simon Fraser University, 8888 University Drive Burnaby, BC, V5A 1S6, Canada * E-mail: [email protected] This work was supported in part by the Natural Sciences and Engineering Research Council of Canada (NSERC; Grant No. RGPIN-2020-06522), and through the Collaborative Health Research Projects (CHRP) Partnership Program supported in part by the Canadian Institutes of Health Research (Grant No. 134742) and the Natural Science Engineering Research Council of Canada (Grant No. CHRP 462260), the Canada Research Chairs Program (B.D. Gates, Grant No. 950-215846), CMC Microsystems (MNT Grant No. 6345), and a Graduate Fellowship (Rana Faryad Ali) from Simon Fraser University. This work made use of 4D LABS (www.4dlabs.com) and the Center for Soft Materials shared facilities supported by the Canada Foundation for Innovation (CFI), British Columbia Knowledge Development Fund (BCKDF), Western Economic Diversification Canada, and Simon Fraser University. S1 Experimental Materials and supplies All chemicals were of analytical grade and were used as received without further purification. Niobium ethoxide [Nb(OC2H5)5, >90%] was obtained from Gelest Inc., and benzyl alcohol (C7H7OH, 99%) and triethylamine [N(C2H5)3, 99.0%] were purchased from Acros Organics and Anachemia, respectively. Lithium chloride (LiCl, ~99.0%) was obtained from BDH Chemicals, and lithium bromide (LiBr, ≥99.0%), lithium fluoride (LiF, ~99.9%), and lithium iodide (LiI, 99.0%) were purchased from Sigma Aldrich. -

Unveiling the Defect Structure of Lithium Niobate with Nuclear Methods
crystals Review Unveiling the Defect Structure of Lithium Niobate with Nuclear Methods Andreas Kling * and José G. Marques Centro de Ciências e Tecnologias Nucleares, Instituto Superior Técnico, Universidade de Lisboa, Estrada Nacional 10, km 139.7, P-2695-066 Bobadela, Portugal; [email protected] * Correspondence: [email protected]; Tel.: +351-219-946-154 Abstract: X-ray and neutron diffraction studies succeeded in the 1960s to determine the principal structural properties of congruent lithium niobate. However, the nature of the intrinsic defects related to the non-stoichiometry of this material remained an object of controversial discussion. In addition, the incorporation mechanism for dopants in the crystal lattice, showing a solubility range from about 0.1 mol% for rare earths to 9 mol% for some elements (e.g., Ti and Mg), stayed unresolved. Various different models for the formation of these defect structures were developed and required experimental verification. In this paper, we review the outstanding role of nuclear physics based methods in the process of unveiling the kind of intrinsic defects formed in congruent lithium niobate and the rules governing the incorporation of dopants. Complementary results in the isostructural compound lithium tantalate are reviewed for the case of the ferroelectric-paraelectric phase transition. We focus especially on the use of ion beam analysis under channeling conditions for the direct determination of dopant lattice sites and intrinsic defects and on Perturbed Angular Correlation measurements probing the local environment of dopants in the host lattice yielding independent and complementary information. Citation: Kling, A.; Marques, J.G. Unveiling the Defect Structure of Keywords: lithium niobate; intrinsic defects; extrinsic defects; lattice location; radiation damage; ion Lithium Niobate with Nuclear beam analysis; hyperfine interactions Methods. -

And Nanoscale Domain Engineering in Lithium Niobate and Lithium Tantalate Vladimir Ya
Micro- and nanoscale domain engineering in lithium niobate and lithium tantalate Vladimir Ya. Shur*a, Evgenii Rumyantsev a Ekaterina Nikolaeva a Eugene Shishkin a b Robert G. Batchko b Gregory D. Miller b Martin M. Fejerb Robert L.Byer alflSt of Phys. & App!. Math., Ural State Univ., Ekaterinburg 620083, Russia bEL Ginzton Laboratory, Stanford University, Stanford, CA 94305-4085 ABSTRACT We present detail investigation of the domain evolution in lithium niobate and lithium tantalate during backswitched electric field poling which allowed to produce micro- and nanoscale domain patterns by application of voltage to lithographically defined strip electrodes. In situ optical observation of the domain kinetics during poling and high-resolution visualization by SEM and SFM of the static domain patterns on polar surfaces and cross-sections have been used. We separated and studied the main stages of domain evolution. The important role of backswitching as a powerfiul tool for high-fidelity domain patterning in thick wafers and for production of quasi-periodic nanoscale domain patterns has been demonstrated. We have proposed several variants of domain manipulation during backswitched poling: the frequency multiplication of the domain patterns, domain "erasing" and "splitting", formation of oriented arrays of nanoscale domains. We have demonstrated the production of lamellar domain patterns with period down to 2.6 microns in 0.5-mm-thick wafers and strictly oriented quasi- periodic domain arrays consisting of the individual nanodomains with diameter down to 30 nm and density up to 100 per square micron. Keywords: domain patterning, backswitchmg, nanotechnology, electric field poling, domain manipulation. 1. INTRODUCTION In recent years rapid development of a new branch of technology named the microdomain engineering raised the questions of fabrication of ferroelectric domain patterns with periods about several microns. -
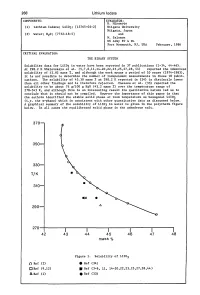
Evaluation of Aqueous Systems
268 Lithium Iodate COMPONENTS: EVALUATOR: H. Miyamoto (1) Lithium Iodate; LiI03; [13765-03-2] Niigata University Niigata, Japan (2) Water; H20; [7732-18-5] and M. Salomon US Army ET & DL Fort Monmouth, NJ, USA February, 1986 CRITICAL EVALUATION: THE BINARY SYSTEM Solubility data for LiI03 in water have been reported in 37 publications (1-34, 44-46). At 298.2 K Shklovskaya et al. (5,7,8,11,14-20,22,23,25,27,28,44) reported the identical solubility of 43.82 mass %, and although the work spans a period of 10 years (1974-1983), it is not possible to determine the number of independent measurements in these 18 publi cations. The solubility of 43.30 mass % at 298.2 K reported in (24) is distinctly lower than all other findings and is therefore rejected. Unezawa et al. (33) reported the solubility to be about 76 g/lOO g H20 (43.2 mass %) over the temperature range of 278-343 K, and although this is an interesting result its qualitative nature led us to conclude that it should not be compiled. However the importance of this paper is that the authors identified the stable solid phase at room temperature as hexagonal LiI03 (i.e. the a-phase) which is consistent with other quantitative data as discussed below. A graphical summary of the solubility of LiI03 in water is given in the polytherm figure below. In all cases the equilibrated solid phase is the anhydrous salt. 370 350 330 T/K 310 290 270-I------.r-----.-----..----......----------. 42 43 44 45 46 47 48 mass % Figure 1. -

Handbook on the Physics and Chemistry of Rare Earths Volume 9 Elsevier, 1987
Handbook on the Physics and Chemistry of Rare Earths volume 9 Elsevier, 1987 Edited by: Karl A. Gschneidner, Jr. and LeRoy Eyring ISBN: 978-0-444-87045-2 by kmno4 Handbook on the Physics and Chemistry of Rare Earths, edited by K.A. Gschneidner, Jr. and L. Eyring © Elsevier Science Publishers B.V., 1987 PREFACE Karl A. GSCHNEIDNER, Jr., and LeRoy EYRING o These elements perplex us in our rearches [sic], baffle us in our speculations, and haunt us in our very dreams. They stretch like an unknown sea before us- mocking, mystifying, and murmuring strange revelations and possibilities. Sir William Crookes (February 16, 1887) There are those who feel that the rare earth elements are destined to play an even greater role in our "high-tech" society in the future than they have in the past. This judgement is based upon the trend of increasing applications resulting from the electronic structures of these materials that lead to their unusual optical, magnetic, electrical and chemical properties so adaptable to the demands now being placed on materials. The "Handbook" seeks to provide topical reviews and compilations of critically reviewed data to aid in the design and fabrication of the required new materials. Four additional chapters in this tradition are the contents of this volume. The development of lasers containing rare earth species sparked an interest in glasses containing these elements. Reisfeld and J0rgensen discuss excited-state phenomena in vitreous rare-earth-containing substances. The description of the chemistry and crystal chemistry of complex inorganic compounds of the rare earths is continued by Niinist6 and Leskelfi from the previous volume. -

Tourmalines and Relaxor Ferroelectrics of The
Correlations between Crystal Structure and Elastic Properties of Piezoelectric Crystals: Tourmalines and Relaxor Ferroelectrics of the type CaxBa1-xNb2O6 and Ce:CaxBa1-xNb2O6 DISSERTATION zur Erlangung des akademischen Grades eines “Doktor der Naturwissenschaften” an der Fakultät für Geowissenschaften der Ruhr-Universität Bochum Germany von Chandra Shekhar Pandey aus Bareilly (India) Bochum, 2010 1. Gutachter Prof. Dr. Jürgen Schreuer 2. Gutachter Prof. Dr. Ladislav Bohatý Datum der Vorlage 20.10.2010 Datum der Disputation 17.12.2010 “If we knew what we were doing, it wouldn't be research” Albert Einstein This thesis is dedicated to My Love Arti & My sweet ‘n’ cute loving daughter Aditi Abstract In this thesis the elastic properties of two distinct groups of piezoelectric crystals are presented: one is natural tourmaline occurring with a vast variability in chemical compositions with a stable phase over a wide temperature range and hence allowing to examine the influence of chemical composition on its physical properties; while the other is synthetically grown relaxor ferroelectric calcium barium niobate (CBN) exhibiting a ferroelectric-paraelectric phase transition and thus invoking the interest to investigate the variation of elastic properties in both phases as well as in the vicinity of the phase transition. To this end the non-destructive innovative method of resonant ultrasound spectroscopy (RUS) was employed. The full sets of elastic, piezoelectric, dielectric constants and coefficients of thermal expansion of five natural single crystal tourmalines of gem quality have been determined between 100 K and 903 K. The main influence of Li and Fe on elastic, piezoelectric and dielectric properties of tourmalines was studied.