Spondylolisthesis.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Transforaminal Endoscopic Discectomy to Relieve Sciatica and Delay Fusion in a 31-Year-Old Man with Pars Defects and Low-Grade Spondylolisthesis
NEUROSURGICAL FOCUS Neurosurg Focus 40 (2):E4, 2016 Transforaminal endoscopic discectomy to relieve sciatica and delay fusion in a 31-year-old man with pars defects and low-grade spondylolisthesis Karthik Madhavan, MD,2 Lee Onn Chieng, BS,2 Christoph P. Hofstetter, MD, PhD,1 and Michael Y. Wang, MD2 1Department of Neurological Surgery, University of Washington, Seattle, Washington; and 2Department of Neurological Surgery and the Miami Project to Cure Paralysis, University of Miami Miller School of Medicine, Miami, Florida Isthmic spondylolisthesis due to pars defects resulting from trauma or spondylolysis is not uncommon. Symptomatic patients with such pars defects are traditionally treated with a variety of fusion surgeries. The authors present a unique case in which such a patient was successfully treated with endoscopic discectomy without iatrogenic destabilization. A 31-year-old man presented with a history of left radicular leg pain along the distribution of the sciatic nerve. He had a disc herniation at L5/S1 and bilateral pars defects with a Grade I spondylolisthesis. Dynamic radiographic studies did not show significant movement of L-5 over S-1. The patient did not desire to have a fusion. After induction of local anesthesia, the patient underwent an awake transforaminal endoscopic discectomy via the extraforaminal approach, with decompression of the L-5 and S-1 nerve roots. His preoperative pain resolved immediately, and he was discharged home the same day. His preoperative Oswestry Disability Index score was 74, and postoperatively it was noted to be 8. At 2-year follow-up he continued to be symptom free, and no radiographic progression of the listhesis was noted. -

Vertebral Column and Thorax
Introduction to Human Osteology Chapter 4: Vertebral Column and Thorax Roberta Hall Kenneth Beals Holm Neumann Georg Neumann Gwyn Madden Revised in 1978, 1984, and 2008 The Vertebral Column and Thorax Sternum Manubrium – bone that is trapezoidal in shape, makes up the superior aspect of the sternum. Jugular notch – concave notches on either side of the superior aspect of the manubrium, for articulation with the clavicles. Corpus or body – flat, rectangular bone making up the major portion of the sternum. The lateral aspects contain the notches for the true ribs, called the costal notches. Xiphoid process – variably shaped bone found at the inferior aspect of the corpus. Process may fuse late in life to the corpus. Clavicle Sternal end – rounded end, articulates with manubrium. Acromial end – flat end, articulates with scapula. Conoid tuberosity – muscle attachment located on the inferior aspect of the shaft, pointing posteriorly. Ribs Scapulae Head Ventral surface Neck Dorsal surface Tubercle Spine Shaft Coracoid process Costal groove Acromion Glenoid fossa Axillary margin Medial angle Vertebral margin Manubrium. Left anterior aspect, right posterior aspect. Sternum and Xyphoid Process. Left anterior aspect, right posterior aspect. Clavicle. Left side. Top superior and bottom inferior. First Rib. Left superior and right inferior. Second Rib. Left inferior and right superior. Typical Rib. Left inferior and right superior. Eleventh Rib. Left posterior view and left superior view. Twelfth Rib. Top shows anterior view and bottom shows posterior view. Scapula. Left side. Top anterior and bottom posterior. Scapula. Top lateral and bottom superior. Clavicle Sternum Scapula Ribs Vertebrae Body - Development of the vertebrae can be used in aging of individuals. -

Brucellar Spondylodiscitis with Rapidly Progressive Spinal Epidural Abscess Showing Cauda Equina Syndrome
Citation: Spinal Cord Series and Cases (2016) 2, 15030; doi:10.1038/scsandc.2015.30 © 2016 International Spinal Cord Society All rights reserved 2058-6124/16 www.nature.com/scsandc CASE REPORT Brucellar spondylodiscitis with rapidly progressive spinal epidural abscess showing cauda equina syndrome Tan Hu1,2,JiWu1,2, Chao Zheng1 and Di Wu1 Early diagnosis of Brucellosis is often difficult in the patient with only single non-specific symptom because of its rarity. We report a patient with Brucellar spondylodiscitis, in which the low back pain was the only symptom and the magnetic resonance imaging (MRI) showed not radiographic features about infection at initial stage. He was misdiagnosed as a lumbar disc herniation for inappropriate treatment in a long time. The delay in diagnosis and correct treatment led to rapid progression of the disease and severe complications. The patient was treated successfully with triple-antibiotic and surgical intervention in the end. Brucellar spondylodiscitis should always be suspended in the differential diagnosis specially when the patient comes from an endemic area or has consumed dairy products from animals in such an area and comprehensive examination should be done for the patent to rule out some important diseases like Brucellosis with sufficient reasons. Spinal Cord Series and Cases (2016) 2, 15030; doi:10.1038/scsandc.2015.30; published online 7 January 2016 Brucellosis is caused by small, non-motile, Gram-negative, aerobic post meridiem and intermittent left lower limb numbness for the and facultative intracellular coccobacilli of the genus Brucella recent weeks. One week before admission, the patient returned transmitted from infected animals to humans either by to the local clinic because his symptoms worsen. -

Tracheal Perforation in a Neonate: a Devastating
Letters to Editor Tracheal perforation in a size of endotracheal tube, cuff overinflation, cough and vigorous movements of head and neck.[2,3] neonate: A devastating Neonatal tracheal injury is ascribed to factors like complication following traumatic traumatic delivery, weak trachea, congenital tracheal endotracheal intubation stenosis, ring agenesis, metal stylets, rigid endotracheal tube, excessive external laryngeal pressure and [1,4,5] Sir, prolonged ventilation. Tracheal injury in neonates following endotracheal In our case, rigorous attempts at intubation along with intubation represents an uncommon complication excessive hyperextension of head and neck due to altered rarely described in literature but carries high anatomy are likely to have contributed to tracheal injury. morbidity and a mortality rate of 70%.[1] We describe Multiple attempts at intubation are known to result in a [4] a case of neonatal tracheal perforation following false tract and are related to anterior tracheal lesions. multiple attempts at endotracheal intubation due to an Incidentally, our case was an undiagnosed Pierre unanticipated difficulty in an emergency situation in Robins Sequence. A small receding mandible, tongue an undiagnosed case of Pierre Robin Sequence. immobility and cleft palate are identified as independent factors for difficult airway with a risk of upper airway A 4-week-old female baby presented to the emergency obstruction, difficult mask holding, difficult intubation, department with respiratory difficulty. In view of severe leak through the cleft resulting in inadequate ventilation respiratory distress and desaturation (SpO2- 60%) a as well as passage of the endotracheal tube into the decision was made to provide ventilatory support to cleft.[6] Successful airway management in such situation the neonate. -

Master.Pmd 2
The Effects of Tumor Necrosis Factor Inhibitors in a Particular Association of Psoriatic Arthritis and Behcet Disease ANCA EMANUELA MUSETESCU1#, ALESANDRA FLORESCU2*, ANA-MARIA BUMBEA3#, LUCIAN MIHAI FLORESCU4, PAULINA LUCIA CIUREA1, ANDREI BONDARI4, DAN NICOLAE FLORESCU5, SIMONA BONDARI4 1 University of Medicine and Pharmacy of Craiova,Department of Rheumatology, 2-4 Petru Rares Str., 200349, Craiova, Romania 2University of Medicine and Pharmacy of Craiova, 2-4 Petru Rares Str., 200349, Craiova, Romania 3University of Medicine and Pharmacy of Craiova, Department of Medical Rehabilitation, 2-4 Petru Rares Str., 200349, Craiova, Romania 4 University of Medicine and Pharmacy of Craiova, Department of Radiology and Medical Imaging, 2-4 Petru Rares Str., 200349, Craiova, Romania 5 University of Medicine and Pharmacy of Craiova, Department of Gastroenterology, 2-4 Petru Rares Str., 200349, Craiova, Romania The current case report presents a patient diagnosed with Behcet disease in concurrence with psoriatic arthritis, leading to a complex treatment, difficult management and challenging approach of both rheumatic disorders. After treatment with synthetic disease-modifying drugs and three different TNF inhibitors, the patient developed pulmonary tuberculosis, followed by tuberculosis spondylodiscitis, even after proper anti- tuberculosis therapy. Keywords: Behcet disease, psoriatic arthritis, tuberculosis spondylodiscitis, TNF inhibitors Psoriatic arthritis (PsA) is a chronic inflammatory disease characterized by the presence of both peripheral of uveitis in patients with BD, except for etanercept with joint and axial spine involvement, in concurrence with conflicting results [5]. psoriatic lesions and in the absence of rheumatoid factor. The term PsA comprises one of the five clinical forms: Experimental part symmetrical polyarthritis, asymmetrical oligoarthritis, distal Case report interphalangeal joint involvement, spondylitis and arthritis We present the case of a 34-year old male admitted in mutilans [1]. -
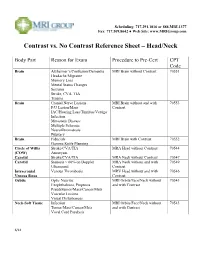
Contrast Vs. No Contrast Reference Sheet – Head/Neck
Scheduling: 717.291.1016 or 888.MRI.1377 Fax: 717.509.8642 ● Web Site: www.MRIGroup.com Contrast vs. No Contrast Reference Sheet – Head/Neck Body Part Reason for Exam Procedure to Pre-Cert CPT Code Brain Alzheimer’s/Confusion/Dementia MRI Brain without Contrast 70551 Headache/Migraine Memory Loss Mental Status Changes Seizures Stroke, CVA, TIA Trauma Brain Cranial Nerve Lesions MRI Brain without and with 70553 F/U Lesion/Mass Contrast IAC/Hearing Loss/Tinnitus/Vertigo Infection Metastatic Disease Multiple Sclerosis Neurofibromatosis Pituitary Brain Fiducials MRI Brain with Contrast 70552 Gamma Knife Planning Circle of Willis Stroke/CVA/TIA MRA Head without Contrast 70544 (COW) Aneurysm Carotid Stroke/CVA/TIA MRA Neck without Contrast 70547 Carotid Stenosis > 60% on Doppler MRA Neck without and with 70549 Ultrasound Contrast Intracranial Venous Thrombosis MRV Head without and with 70546 Venous Sinus Contrast Orbits Optic Neuritis MRI Orbits/Face/Neck without 70543 Exophthalmos, Proptosis and with Contrast Pseudotumor/Mass/Cancer/Mets Vascular Lesions Visual Disturbances Neck-Soft Tissue Infection MRI Orbits/Face/Neck without 70543 Tumor/Mass/Cancer/Mets and with Contrast Vocal Cord Paralysis 6/14 Scheduling: 717.291.1016 or 888.MRI.1377 Fax: 717.509.8642 ● Web Site: www.MRIGroup.com Contrast vs. No Contrast Reference Sheet – Spine Body Part Reason for Exam Procedure to Pre-Cert CPT Code Spine: Cervical Degenerative Disease MRI Cervical Spine without Contrast 72141 Disc Herniation Extremity Pain/Weakness Neck Pain Radiculopathy Trauma Spine: -
Spinal Deformity Study Group
Spinal Deformity Study Group Editors in Chief Radiographic Michael F. O’Brien, MD Timothy R. Kuklo, MD Kathy M. Blanke, RN Measurement Lawrence G. Lenke, MD Manual B T2 T5 T2–T12 CSVL T5–T12 +X° -X +X° C7PL T12 L2 A S1 ©2008 Medtronic Sofamor Danek USA, Inc. – 0 + Radiographic Measurement Manual Editors in Chief Michael F. O’Brien, MD Timothy R. Kuklo, MD Kathy M. Blanke, RN Lawrence G. Lenke, MD Section Editors Keith H. Bridwell, MD Kathy M. Blanke, RN Christopher L. Hamill, MD William C. Horton, MD Timothy R. Kuklo, MD Hubert B. Labelle, MD Lawrence G. Lenke, MD Michael F. O’Brien, MD David W. Polly Jr, MD B. Stephens Richards III, MD Pierre Roussouly, MD James O. Sanders, MD ©2008 Medtronic Sofamor Danek USA, Inc. Acknowledgements Radiographic Measurement Manual The radiographic measurement manual has been developed to present standardized techniques for radiographic measurement. In addition, this manual will serve as a complimentary guide for the Spinal Deformity Study Group’s radiographic measurement software. Special thanks to the following members of the Spinal Deformity Study Group in the development of this manual. Sigurd Berven, MD Hubert B. Labelle, MD Randal Betz, MD Lawrence G. Lenke, MD Fabien D. Bitan, MD Thomas G. Lowe, MD John T. Braun, MD John P. Lubicky, MD Keith H. Bridwell, MD Steven M. Mardjetko, MD Courtney W. Brown, MD Richard E. McCarthy, MD Daniel H. Chopin, MD Andrew A. Merola, MD Edgar G. Dawson, MD Michael Neuwirth, MD Christopher DeWald, MD Peter O. Newton, MD Mohammad Diab, MD Michael F. -

Lordosis, Kyphosis, and Scoliosis
SPINAL CURVATURES: LORDOSIS, KYPHOSIS, AND SCOLIOSIS The human spine normally curves to aid in stability or balance and to assist in absorbing shock during movement. These gentle curves can be seen from the side or lateral view of the spine. When viewed from the back, the spine should run straight down the middle of the back. When there are abnormalities or changes in the natural spinal curvature, these abnormalities are named with the following conditions and include the following symptoms. LORDOSIS Some lordosis is normal in the lower portion or, lumbar section, of the human spine. A decreased or exaggerated amount of lordosis that is causing spinal instability is a condition that may affect some patients. Symptoms of Lordosis include: ● Appearance of sway back where the lower back region has a pronounced curve and looks hollow with a pronounced buttock area ● Difficulty with movement in certain directions ● Low back pain KYPHOSIS This condition is diagnosed when the patient has a rounded upper back and the spine is bent over or curved more than 50 degrees. Symptoms of Kyphosis include: ● Curved or hunched upper back ● Patient’s head that leans forward ● May have upper back pain ● Experiences upper back discomfort after movement or exercise SCOLIOSIS The most common of the three curvatures. This condition is diagnosed when the spine looks like a “s” or “c” from the back. The spine is not straight up and down but has a curve or two running side-to-side. Sagittal Balance Definition • Sagittal= front-to-back direction (sagittal plane) • Imbalance= Lack of harmony or balance Etiology • Excessive lordosis (backwards lean) or kyphosis (forward lean) • Traumatic injury • Previous spinal fusion that disrupted sagittal balance Effects • Low back pain • Difficulty walking • Inability to look straight ahead when upright The most ergonomic and natural posture is to maintain neutral balance, with the head positioned over the shoulders and pelvis. -

Case Report Spinal Gout with Lumbar Spondylolisthesis: Case Report and Review of the Literature
Int J Clin Exp Med 2017;10(3):5493-5496 www.ijcem.com /ISSN:1940-5901/IJCEM0043949 Case Report Spinal gout with lumbar spondylolisthesis: case report and review of the literature Hui Zhang, Wenhao Zheng, Naifeng Tian, Xiangyang Wang, Yan Lin, Yaosen Wu Department of Orthopaedic Surgery, The Second Affiliated Hospital and Yuying Children’s Hospital of Wenzhou Medical University, Wenzhou, China Received November 9, 2016; Accepted December 30, 2016; Epub March 15, 2017; Published March 30, 2017 Abstract: Gout, a metabolic disorder, is commonly accepted as a peripheral joint disease of the appendicular skel- eton by the deposition of monosodium urate crystals. Gouty involvement of the spinal column is rare. In this paper, we report a case of spinal gout with spondylolisthesis, meanwhile, we review the clinical, radiological features, diagnosis and treatment of spinal gout in literature. The patient was 60-year-old with low back pain. Radiological examinations of the lumbar spine showed L4 spondylolisthesis with bone erosion in facet joints and lamina. The patient was treated by L4/5 transforaminal lumbar interbody fusion. The postoperative histological examination confirmed the diagnosis of spinal gout. Spinal gout is rare and can easily be underestimated. Clinician should keep in mind spinal gout as a differential diagnosis especially in patients with long history of gout and axial symptoms. Keywords: Spine, gout, low back pain, spondylolisthesis, cord compression, computed tomography Introduction treatment with enalapril and indapamide are used to control hypertension. However, there is Gout is a common metabolic disorder which is no effective treatment of gout. characterized by precipitation of urate crystals in joints and soft tissue. -

Diaphragm and Intercostal Muscles
Diaphragm and intercostal muscles Dr. Heba Kalbouneh Associate Professor of Anatomy and Histology Skeletal System Adult Human contains 206 Bones 2 parts: Axial skeleton (axis): Skull, Vertebral column, Thoracic cage Appendicular skeleton: Bones of upper limb Bones of lower limb Dr. Heba Kalbouneh Structure of Typical Vertebra Body Vertebral foramen Pedicle Transverse process Spinous process Lamina Dr. Heba Kalbouneh Superior articular process Intervertebral disc Dr. Heba Inferior articular process Dr. Heba Facet joints are between the superior articular process of one vertebra and the inferior articular process of the vertebra directly above it Inferior articular process Superior articular process Dr. Heba Kalbouneh Atypical Vertebrae Atlas (1st cervical vertebra) Axis (2nd cervical vertebra) Dr. Heba Atlas (1st cervical vertebra) Communicates: sup: skull (atlanto-occipital joint) inf: axis (atlanto-axial joint) Atlas (1st cervical vertebra) Characteristics: 1. no body 2. no spinous process 3. ant. & post. arches 4. 2 lateral masses 5. 2 transverse foramina Typical cervical vertebra Specific to the cervical vertebra is the transverse foramen (foramen transversarium). is an opening on each of the transverse processes which gives passage to the vertebral artery Thoracic Cage - Sternum (G, sternon= chest bone) -12 pairs of ribs & costal cartilages -12 thoracic vertebrae Manubrium Body Sternum: Flat bone 3 parts: Xiphoid process Dr. Heba Kalbouneh Dr. Heba Kalbouneh The external intercostal muscle forms the most superficial layer. Its fibers are directed downward and forward from the inferior border of the rib above to the superior border of the rib below The muscle extends forward to the costal cartilage where it is replaced by an aponeurosis, the anterior (external) intercostal membrane Dr. -

Spinal Cord Stimulation in Failed Back Surgery Syndrome: Effects on Posture and Gait—A Preliminary 3D Biomechanical Study
Hindawi Pain Research and Management Volume 2017, Article ID 3059891, 9 pages https://doi.org/10.1155/2017/3059891 Research Article Spinal Cord Stimulation in Failed Back Surgery Syndrome: Effects on Posture and Gait—A Preliminary 3D Biomechanical Study L. Brugliera,1 A. De Luca,2 S. Corna,1 M. Bertolotto,3 G. A. Checchia,4 M. Cioni,5 P. Capodaglio,1 and C. Lentino2,4 1 Istituto Auxologico Italiano, Unita` di Riabilitazione Osteoarticolare, Ospedale S Giuseppe, Strada Cadorna 90, Piancavallo, Italy 2Laboratorio Analisi del Movimento Ospedale Santa Corona di Pietra Ligure, Viale 25 Aprile 38, 17027 Pietra Ligure, Italy 3Centro Terapia del Dolore e Cure Palliative, Dipartimento di Riabilitazione, Ospedale Santa Corona di Pietra Ligure, Viale 25 Aprile 38, 17027 Pietra Ligure, Italy 4Struttura Complessa Recupero e Rieducazione Funzionale, Ospedale Santa Corona di Pietra Ligure, Viale 25 Aprile 38, 17027 Pietra Ligure, Italy 5Scuola di Specializzazione in Medicina Fisica e Riabilitativa, Dipartimento di Scienze Biomediche e Biotecnologiche, Universita` degli Studi di Catania, Piazza Universita2,95131Catania,Italy` Correspondence should be addressed to P. Capodaglio; [email protected] Received 30 March 2017; Revised 21 June 2017; Accepted 1 August 2017; Published 25 September 2017 Academic Editor: Emily J. Bartley Copyright © 2017 L. Brugliera et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. We studied 8 patients with spinal cord stimulation (SCS) devices which had been previously implanted to treat neuropathic chronic pain secondary to Failed Back Surgery Syndrome. -

5. Vertebral Column
5. Vertebral Column. Human beings belong to a vast group animals, the vertebrates. In simple terms we say that vertebrates are animals with a backbone. This statement barely touches the surface of the issue. Vertebrates are animals with a bony internal skeleton. Besides, all vertebrates have a fundamental common body plan. The central nervous system is closer to the back than it is to the belly, the digestive tube in the middle and the heart is ventral. The body is made of many segments (slices) built to a common plan, but specialised in different regions of the body. A “coelomic cavity” with its own special plan is seen in the trunk region and has a characteristic relationship with the organs in the trunk. This is by no means a complete list of vertebrate characteristics. Moreover, some of these features may be shared by other animal groups in a different manner. Such a study is beyond the scope of this unit. Vertebrates belong to an even wider group of animals, chordates. It may be difficult to imagine that we human beings are in fact related to some the earlier chordates! However, we do share, at least during embryonic development, an important anatomical structure with all chordates. This structure is the notochord. The notochord is the first stiff, internal support that appeared during the evolutionary story. As we have seen in early embryology, it also defines the axis of the body. The vertebral column evolved around the notochord and the neural tube, and we see a reflection of this fact during our embryonic development.