Procedure Guideline for Breast Scintigraphy
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

BREAST IMAGING for SCREENING and DIAGNOSING CANCER Policy Number: DIAGNOSTIC 105.9 T2 Effective Date: January 1, 2017
Oxford UnitedHealthcare® Oxford Clinical Policy BREAST IMAGING FOR SCREENING AND DIAGNOSING CANCER Policy Number: DIAGNOSTIC 105.9 T2 Effective Date: January 1, 2017 Table of Contents Page Related Policies INSTRUCTIONS FOR USE .......................................... 1 Omnibus Codes CONDITIONS OF COVERAGE ...................................... 1 Preventive Care Services BENEFIT CONSIDERATIONS ...................................... 2 Radiology Procedures Requiring Precertification for COVERAGE RATIONALE ............................................. 3 eviCore Healthcare Arrangement APPLICABLE CODES ................................................. 5 DESCRIPTION OF SERVICES ...................................... 6 CLINICAL EVIDENCE ................................................. 7 U.S. FOOD AND DRUG ADMINISTRATION ................... 16 REFERENCES .......................................................... 18 POLICY HISTORY/REVISION INFORMATION ................ 22 INSTRUCTIONS FOR USE This Clinical Policy provides assistance in interpreting Oxford benefit plans. Unless otherwise stated, Oxford policies do not apply to Medicare Advantage members. Oxford reserves the right, in its sole discretion, to modify its policies as necessary. This Clinical Policy is provided for informational purposes. It does not constitute medical advice. The term Oxford includes Oxford Health Plans, LLC and all of its subsidiaries as appropriate for these policies. When deciding coverage, the member specific benefit plan document must be referenced. The terms -

Breast Scintimammography
CLINICAL MEDICAL POLICY Policy Name: Breast Scintimammography Policy Number: MP-105-MD-PA Responsible Department(s): Medical Management Provider Notice Date: 11/23/2020 Issue Date: 11/23/2020 Effective Date: 12/21/2020 Next Annual Review: 10/2021 Revision Date: 09/16/2020 Products: Gateway Health℠ Medicaid Application: All participating hospitals and providers Page Number(s): 1 of 5 DISCLAIMER Gateway Health℠ (Gateway) medical policy is intended to serve only as a general reference resource regarding coverage for the services described. This policy does not constitute medical advice and is not intended to govern or otherwise influence medical decisions. POLICY STATEMENT Gateway Health℠ does not provide coverage in the Company’s Medicaid products for breast scintimammography. The service is considered experimental and investigational in all applications, including but not limited to use as an adjunct to mammography or in staging the axillary lymph nodes. This policy is designed to address medical necessity guidelines that are appropriate for the majority of individuals with a particular disease, illness or condition. Each person’s unique clinical circumstances warrant individual consideration, based upon review of applicable medical records. (Current applicable Pennsylvania HealthChoices Agreement Section V. Program Requirements, B. Prior Authorization of Services, 1. General Prior Authorization Requirements.) Policy No. MP-105-MD-PA Page 1 of 5 DEFINITIONS Prior Authorization Review Panel – A panel of representatives from within the Pennsylvania Department of Human Services who have been assigned organizational responsibility for the review, approval and denial of all PH-MCO Prior Authorization policies and procedures. Scintimammography A noninvasive supplemental diagnostic testing technology that requires the use of radiopharmaceuticals in order to detect tissues within the breast that accumulate higher levels of radioactive tracer that emit gamma radiation. -

A Molecular Approach to Breast Imaging
Journal of Nuclear Medicine, published on January 16, 2014 as doi:10.2967/jnumed.113.126102 FOCUS ON MOLECULAR IMAGING A Molecular Approach to Breast Imaging Amy M. Fowler Department of Radiology, University of Wisconsin–Madison, Madison, Wisconsin malignant cells. A recent meta-analysis of the accuracy of 99mTc-sestamibi scintimammography as an adjunct to di- Molecular imaging is a multimodality discipline for noninvasively agnostic mammography for detection of breast cancer dem- visualizing biologic processes at the subcellular level. Clinical applications of radionuclide-based molecular imaging for breast onstrated a sensitivity of 83% and specificity of 85% (2). cancer continue to evolve. Whole-body imaging, with scinti- However, sensitivity was less for nonpalpable (59%) versus mammography and PET, and newer dedicated breast imaging palpable lesions (87%) despite comparable specificity, with systems are reviewed. The potential clinical indications and the no significant difference between planar and SPECT meth- challenges of implementing these emerging technologies are ods. Decreased sensitivity for nonpalpable, presumably presented. smaller, lesions is in part due to the limited spatial resolu- Key Words: molecular imaging; oncology; breast; PET; PET/ tion of conventional g cameras. CT; radiopharmaceuticals; breast cancer; breast-specific g im- In addition to 99mTc-sestamibi, the positron-emitting ra- aging; positron-emission mammography; positron-emission to- diopharmaceutical 18F-FDG accumulates in many types of mography cancer including breast. Meta-analyses of the accuracy of J Nucl Med 2014; 55:1–4 whole-body 18F-FDG PET used after standard diagnostic DOI: 10.2967/jnumed.113.126102 workup for patients with suspected breast lesions demon- strated sensitivities of 83%–89% and specificities of 74%– 80% (3,4). -

Evaluation of Nipple Discharge
New 2016 American College of Radiology ACR Appropriateness Criteria® Evaluation of Nipple Discharge Variant 1: Physiologic nipple discharge. Female of any age. Initial imaging examination. Radiologic Procedure Rating Comments RRL* Mammography diagnostic 1 See references [2,4-7]. ☢☢ Digital breast tomosynthesis diagnostic 1 See references [2,4-7]. ☢☢ US breast 1 See references [2,4-7]. O MRI breast without and with IV contrast 1 See references [2,4-7]. O MRI breast without IV contrast 1 See references [2,4-7]. O FDG-PEM 1 See references [2,4-7]. ☢☢☢☢ Sestamibi MBI 1 See references [2,4-7]. ☢☢☢ Ductography 1 See references [2,4-7]. ☢☢ Image-guided core biopsy breast 1 See references [2,4-7]. Varies Image-guided fine needle aspiration breast 1 Varies *Relative Rating Scale: 1,2,3 Usually not appropriate; 4,5,6 May be appropriate; 7,8,9 Usually appropriate Radiation Level Variant 2: Pathologic nipple discharge. Male or female 40 years of age or older. Initial imaging examination. Radiologic Procedure Rating Comments RRL* See references [3,6,8,10,13,14,16,25- Mammography diagnostic 9 29,32,34,42-44,71-73]. ☢☢ See references [3,6,8,10,13,14,16,25- Digital breast tomosynthesis diagnostic 9 29,32,34,42-44,71-73]. ☢☢ US is usually complementary to mammography. It can be an alternative to mammography if the patient had a recent US breast 9 mammogram or is pregnant. See O references [3,5,10,12,13,16,25,30,31,45- 49]. MRI breast without and with IV contrast 1 See references [3,8,23,24,35,46,51-55]. -

Evaluation of the Quantitative Accuracy of a Commercially-Available Positron Emission Mammography Scanner
The Texas Medical Center Library DigitalCommons@TMC The University of Texas MD Anderson Cancer Center UTHealth Graduate School of The University of Texas MD Anderson Cancer Biomedical Sciences Dissertations and Theses Center UTHealth Graduate School of (Open Access) Biomedical Sciences 8-2010 EVALUATION OF THE QUANTITATIVE ACCURACY OF A COMMERCIALLY-AVAILABLE POSITRON EMISSION MAMMOGRAPHY SCANNER Adam Springer Follow this and additional works at: https://digitalcommons.library.tmc.edu/utgsbs_dissertations Part of the Diagnosis Commons, Equipment and Supplies Commons, and the Other Medical Sciences Commons Recommended Citation Springer, Adam, "EVALUATION OF THE QUANTITATIVE ACCURACY OF A COMMERCIALLY-AVAILABLE POSITRON EMISSION MAMMOGRAPHY SCANNER" (2010). The University of Texas MD Anderson Cancer Center UTHealth Graduate School of Biomedical Sciences Dissertations and Theses (Open Access). 64. https://digitalcommons.library.tmc.edu/utgsbs_dissertations/64 This Thesis (MS) is brought to you for free and open access by the The University of Texas MD Anderson Cancer Center UTHealth Graduate School of Biomedical Sciences at DigitalCommons@TMC. It has been accepted for inclusion in The University of Texas MD Anderson Cancer Center UTHealth Graduate School of Biomedical Sciences Dissertations and Theses (Open Access) by an authorized administrator of DigitalCommons@TMC. For more information, please contact [email protected]. EVALUATION OF THE QUANTITATIVE ACCURACY OF A COMMERCIALLY- AVAILABLE POSITRON EMISSION MAMMOGRAPHY SCANNER -
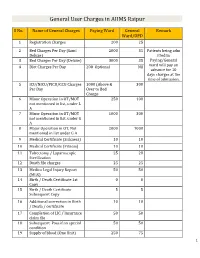
General User Charges in AIIMS Raipur
General User Charges in AIIMS Raipur S No. Name of General Charges Paying Ward General Remark Ward/OPD 1 Registration Charges 200 25 2 Bed Charges Per Day (Sami 2000 35 Patients being adm Deluxe) itted in 3 Bed Charges Per Day (Deluxe) 3000 35 Paying/General 4 Diet Charges Per Day 200 Optional Nil ward will pay an advance for 10 days charges at the time of admission. 5 ICU/NICU/PICU/CCU Charges 1000 (Above & 300 Per Day Over to Bed Charge 6 Minor Operation in OT/MOT 250 100 not mentioned in list, under L A 7 Minor Operation in OT/MOT 1000 300 not mentioned in list, under G A 8 Major Operation in OT, Not 2000 1000 mentioned in list under G A 9 Medical Certificate (Sickness) 10 10 10 Medical Certificate (Fitness) 10 10 11 Tubectomy / Laparoscopic 25 20 Sterilization 12 Death file charges 25 25 13 Medico Legal Injury Report 50 50 (MLR) 14 Birth / Death Certificate 1st 0 0 Copy 15 Birth / Death Certificate 5 5 Subsequent Copy 16 Additional correction in Birth 10 10 / Death / certificate 17 Completion of LIC / Insurance 50 50 claim file 18 Subsequent Pass if on special 50 50 condition 19 Supply of blood (One Unit) 250 75 1 20 Medical Board Certificate 500 500 On Special Case User Charges for Investigations in AIIMS Raipur S No. Name of Investigations Paying General Remark Ward Ward/OPD Anaesthsia 1 ABG 75 50 2 ABG ALONGWITH 150 100 ELECTROLYTES(NA+,K+)(Na,K) 3 ONLY ELECTROLYTES(Na+,K+,Cl,Ca+) 75 50 4 ONLY CALCIUM 50 25 5 GLUCOSE 25 20 6 LACTATE 25 20 7 UREA. -

Scintimammography and Gamma Imaging of the Breast and Axilla
MEDICAL POLICY POLICY TITLE SCINTIMAMMOGRAPHY AND GAMMA IMAGING OF THE BREAST AND AXILLA POLICY NUMBER MP-5.021 Original Issue Date (Created): 8/9/2002 Most Recent Review Date (Revised): 8/18/2020 Effective Date: 11/1/2020 POLICY PRODUCT VARIATIONS DESCRIPTION/BACKGROUND RATIONALE DEFINITIONS BENEFIT VARIATIONS DISCLAIMER CODING INFORMATION REFERENCES POLICY HISTORY I. POLICY Scintimammography, breast-specific gamma imaging (BSGI), and molecular breast imaging (MBI) are considered investigational in all applications, including but not limited to its use as an adjunct to mammography or in staging the axillary lymph nodes. There is insufficient evidence to support a conclusion concerning the health outcomes or benefits associated with these procedures. Use of gamma detection following radiopharmaceutical administration for localization of sentinel lymph nodes in patients with breast cancer may be considered medically necessary. Policy Guidelines The most commonly-used radiopharmaceutical in breast-specific gamma imaging or molecular breast imaging is technetium 99m (Tc-99m) sestamibi. The most commonly used radiopharmaceuticals for sentinel lymph node detection using either lymphoscintigraphy or hand-held gamma detection include Tc 99mlabeled colloids (eg, sulfur colloid). Cross-references: MP-5.022 Radioimmunoscintigraphy Imaging Monoclonal Antibody Imaging with Indium- 111 Capromab Pendetide for Prostate Cancer II. PRODUCT VARIATIONS Top This policy is only applicable to certain programs and products administered by Capital BlueCross please see additional information below, and subject to benefit variations as discussed in Section VI below. Page 1 MEDICAL POLICY POLICY TITLE SCINTIMAMMOGRAPHY AND GAMMA IMAGING OF THE BREAST AND AXILLA POLICY NUMBER MP-5.021 III. DESCRIPTION/BACKGROUND Top Scintimammography, breast-specific gamma imaging (BSGI), and molecular breast imaging (MBI) use radiotracers with nuclear medicine imaging as a diagnostic tool for abnormalities of the breast. -

Scintimammography with a Hybrid SPECT/CT Imaging System
ANTICANCER RESEARCH 27: 557-562 (2007) Scintimammography with a Hybrid SPECT/CT Imaging System ORAZIO SCHILLACI, ROBERTA DANIELI, LUCA FILIPPI, PASQUALE ROMANO, ELSA COSSU, CARLO MANNI and GIOVANNI SIMONETTI Department of Biopathology and Diagnostic Imaging, University "Tor Vergata", Rome, Italy Abstract. Background: Planar scintimammography is useful carcinomas are evident on mammograms, especially in dense for characterizing breast lesions >10 mm. Our aim was to or dysplastic breasts (3); moreover, its specificity and positive evaluate Tc-99m sestamibi scintimammography with a hybrid predictive value are low because it cannot always (SPECT/CT) device for functional anatomical mapping (FAM). differentiate benign lesions from malignant ones (4). The Patients and Methods: Three planar images and a chest drawbacks of mammography have led to the development of SPECT/CT were performed with a hybrid device in 53 patients complementary modalities for breast cancer imaging, with mammographically suspicious lesions. The final including scintimammography, a nuclear medicine technique histopathological diagnosis was obtained after surgery. Results: that uses radiopharmaceuticals to detect malignant breast The planar images were positive in 27 out of 37 carcinomas tumours (5, 6). Scintimammography is conventionally (sensitivity 73%) and the SPECT/CT in 33 (sensitivity 89.2%). performed with planar acquisitions, which have a low The sensitivity of planar imaging and SPECT/CT was 42.9% sensitivity for lesions ≤10 mm (5). Recently, a new imaging and 71.4% in cancers ≤10 mm, and 91.3%, and 100% in device combining a dual-head, variable angle gamma camera cancers >10 mm, respectively. The specificity was 93.8% for with a low-dose X-ray tube has been introduced (7): this both planar and SPECT/CT imaging; accuracy was 79.2% for hybrid gamma camera/CT scanner provides cross-sectional planar scans and 90.6% for SPECT/CT. -

SNMMI Nuclear Medicine Technology Competency Based Curriculum
Nuclear Medicine Technology Competency- Based Curriculum Guide 5th Edition Introduction Competency-based education in nuclear medicine technology focuses on those elements necessary to become an entry-level nuclear medicine technologist. Emphasizing competencies communicates entry-level knowledge, skills, attitudes, and behaviors that the nuclear medicine technology curriculum must address and that employers can expect of graduates. Competency- based education allows more flexibility in pedagogical approaches to achieve these essential competencies. The competencies are divided into eight sections: 1. Radiation Safety 2. Instrumentation, Quality Control and Quality Assurance 3. Radiopharmacy and Pharmacology 4. Diagnostic and Therapeutic Procedures 5. Patient Care 6. Professionalism and Interpersonal Communication Skills 7. Organization Systems-Based Practice 8. Research Methodology Each section lists the competencies that must be achieved by the entry level nuclear medicine technologist. The rigor of the nuclear medicine technologist entry-level competencies is such that a significant body of knowledge, skills, and experience is necessary to achieve them. This level of rigor is consistent with the Society of Nuclear Medicine and Molecular Imaging—Technologist Section’s (SNMMI-TS) recommendation that the entry-level degree for a nuclear medicine technologist should be at the baccalaureate level. The content listed under the competencies is intended to be used as a guide for what may be included in a program’s curriculum to achieve minimal competency in each area. The specific content listed should be used at the program’s discretion to meet individual curricular and accreditation needs. The content in its entirety is not considered mandatory, and other pedagogies may equally meet each program’s needs. -

Infrequently Performed Studies in Nuclear Medicine: Part 1
Infrequently Performed Studies in Nuclear Medicine: Part 1 Anita MacDonald and Steven Burrell Department of Diagnostic Radiology, Queen Elizabeth II Health Sciences Centre and Dalhousie University, Halifax, Nova Scotia, Canada clinical and technical aspects of each study are discussed, Nuclear medicine is a diverse field with a large number of differ- along with a brief comparison of alternative assessment ent studies spanning virtually all organ systems and medical spe- modalities. Included in Part 1 of this article are dacroscin- cialties. Many nuclear medicine procedures are performed tigraphy, LeVeen shunts, scintimammography, right-to-left routinely; others may be performed only rarely, sometimes less (R-L) shunts, left-to-right (L-R) shunts, and heat-damaged than once per year. The infrequent nature of many studies makes it challenging to retain relevant knowledge and skills. This 2-part red blood cell (RBC) studies. Part 2 will cover cerebral article provides a review of several infrequently performed stud- spinal fluid shunt, brain death, testicular scan, quantitative ies. The topics discussed in Part 1 include dacroscintigraphy, lung perfusion scan, lymphoscintigraphy, and salivary gland LeVeen shunts, scintimammography, right-to-left shunts, left- scintigraphy studies. to-right shunts, and heat-damaged red blood cells. After reading this article, the reader should be able to list and describe the in- DACROSCINTIGRAPHY (LACRIMAL GLAND STUDY) dications for each study, list the doses and describe their proper method of administration, and describe problems that may arise The lacrimal glands are located in the lateral superior during the imaging procedure and how they should be handled. portion of each orbit. -

Society of Nuclear Medicine Procedure Guideline for Breast Scintigraphy Version 2.0, Approved June 2, 2004
Society of Nuclear Medicine Procedure Guideline for Breast Scintigraphy Version 2.0, approved June 2, 2004 Authors: Iraj Khalkhali, MD (Harbor-UCLA Medical Center, Torrance, CA); Gina Caravaglia, MD (Harbor-UCLA Medical Center, Torrance, CA); Hani H. Abdel-Nabi, MD, PhD (University of Buffalo, Buffalo, NY); Patrick J. Peller, MD (Woodburn Nuclear Medicine, Annandale, VA); Raymond Taillefer, MD (Hospital Hotel-Dieu de Montreal, Montreal, Quebec, Canada); Penny R. Vande Streek, DO (Sutter Roseville Medical Center, Roseville, CA); and Christophe Van de Wiele, MD (Universitair Ziekenhuis, Ghent, Belgium). I. Purpose carcinomas in patients with tissue diagnosis of breast cancer. The purpose of this guideline is to assist nuclear C. May be useful in the evaluation of the effective- medicine practitioners in recommending, performing, 99m ness of neoadjuvant chemotherapy for breast car- interpreting, and reporting the results of Tc- cinoma. sestamibi breast scintigraphy (mammoscintigraphy, scintimammography). IV. Procedure II. Background Information and Definitions A. Patient Preparation 1. No special preparation for the test is needed; Breast scintigraphy is performed after intravenous 99m however, a thorough explanation of the test administration of Tc-sestamibi and includes planar should be provided by the technologist or and/or single-photon emission computed tomography physician. Before radiopharmaceutical injec- (SPECT). tion, the technologist may have the patient at- tempt the prone position with arms extended, III. Examples of Clinical or Research to assess the feasibility of the study. Applications 2. The patient should remove all clothing and jewelry above the waist and should wear a A. Evaluate breast cancer in patients in whom hospital gown open in front. -

BREAST IMAGING for SCREENING and DIAGNOSING CANCER Policy Number: DIAGNOSTIC 105.14 T2 Effective Date: January 1, 2018
UnitedHealthcare® Oxford Clinical Policy BREAST IMAGING FOR SCREENING AND DIAGNOSING CANCER Policy Number: DIAGNOSTIC 105.14 T2 Effective Date: January 1, 2018 Table of Contents Page Related Policies INSTRUCTIONS FOR USE .......................................... 1 Omnibus Codes CONDITIONS OF COVERAGE ...................................... 1 Preventive Care Services BENEFIT CONSIDERATIONS ...................................... 2 Radiology Procedures Requiring Precertification for COVERAGE RATIONALE ............................................. 3 eviCore healthcare Arrangement APPLICABLE CODES ................................................. 4 DESCRIPTION OF SERVICES ...................................... 5 CLINICAL EVIDENCE ................................................. 6 U.S. FOOD AND DRUG ADMINISTRATION ................... 12 REFERENCES .......................................................... 13 POLICY HISTORY/REVISION INFORMATION ................ 16 INSTRUCTIONS FOR USE This Clinical Policy provides assistance in interpreting Oxford benefit plans. Unless otherwise stated, Oxford policies do not apply to Medicare Advantage members. Oxford reserves the right, in its sole discretion, to modify its policies as necessary. This Clinical Policy is provided for informational purposes. It does not constitute medical advice. The term Oxford includes Oxford Health Plans, LLC and all of its subsidiaries as appropriate for these policies. When deciding coverage, the member specific benefit plan document must be referenced. The terms of