Evaluation of the Quantitative Accuracy of a Commercially-Available Positron Emission Mammography Scanner
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

BREAST IMAGING for SCREENING and DIAGNOSING CANCER Policy Number: DIAGNOSTIC 105.9 T2 Effective Date: January 1, 2017
Oxford UnitedHealthcare® Oxford Clinical Policy BREAST IMAGING FOR SCREENING AND DIAGNOSING CANCER Policy Number: DIAGNOSTIC 105.9 T2 Effective Date: January 1, 2017 Table of Contents Page Related Policies INSTRUCTIONS FOR USE .......................................... 1 Omnibus Codes CONDITIONS OF COVERAGE ...................................... 1 Preventive Care Services BENEFIT CONSIDERATIONS ...................................... 2 Radiology Procedures Requiring Precertification for COVERAGE RATIONALE ............................................. 3 eviCore Healthcare Arrangement APPLICABLE CODES ................................................. 5 DESCRIPTION OF SERVICES ...................................... 6 CLINICAL EVIDENCE ................................................. 7 U.S. FOOD AND DRUG ADMINISTRATION ................... 16 REFERENCES .......................................................... 18 POLICY HISTORY/REVISION INFORMATION ................ 22 INSTRUCTIONS FOR USE This Clinical Policy provides assistance in interpreting Oxford benefit plans. Unless otherwise stated, Oxford policies do not apply to Medicare Advantage members. Oxford reserves the right, in its sole discretion, to modify its policies as necessary. This Clinical Policy is provided for informational purposes. It does not constitute medical advice. The term Oxford includes Oxford Health Plans, LLC and all of its subsidiaries as appropriate for these policies. When deciding coverage, the member specific benefit plan document must be referenced. The terms -

Future of Breast Elastography
Future of breast elastography Richard Gary Barr1,2 1Department of Radiology, Northeastern Ohio Medical University, Rootstown, OH; 2Southwoods Imaging, Youngstown, OH, USA REVIEW ARTICLE Both strain elastography and shear wave elastography have been shown to have high sensitivity https://doi.org/10.14366/usg.18053 pISSN: 2288-5919 • eISSN: 2288-5943 and specificity for characterizing breast lesions as benign or malignant. Training is important for Ultrasonography 2019;38:93-105 both strain and shear wave elastography. The unique feature of benign lesions measuring smaller on elastography than B-mode imaging and malignant lesions appearing larger on elastography is an important feature for characterization of breast masses. There are several artifacts which can contain diagnostic information or alert to technique problems. Both strain and shear wave elastography continue to have improvements and new techniques will soon be available for Received: September 21, 2018 clinical use that may provide additional diagnostic information. This paper reviews the present Revised: January 4, 2019 Accepted: January 4, 2019 state of breast elastography and discusses future techniques that are not yet in clinical practice. Correspondence to: Richard Gary Barr, MD, PhD, Keywords: Breast; Elasticity imaging techniques; Strain; Shear wave; Strain ratio; Southwoods Imaging, 7623 Market Street, Youngstown, OH 44512, USA Breast neoplasms Tel. +1-330-965-5100 Fax. +1-330-965-5109 E-mail: [email protected] Introduction The use of palpation to determine the stiffness of a lesion has been used since the time of the ancient Greeks and Egyptians [1]. Stiff, non-mobile lesions of the breast have a high probability of being malignant. -

Breast Scintimammography
CLINICAL MEDICAL POLICY Policy Name: Breast Scintimammography Policy Number: MP-105-MD-PA Responsible Department(s): Medical Management Provider Notice Date: 11/23/2020 Issue Date: 11/23/2020 Effective Date: 12/21/2020 Next Annual Review: 10/2021 Revision Date: 09/16/2020 Products: Gateway Health℠ Medicaid Application: All participating hospitals and providers Page Number(s): 1 of 5 DISCLAIMER Gateway Health℠ (Gateway) medical policy is intended to serve only as a general reference resource regarding coverage for the services described. This policy does not constitute medical advice and is not intended to govern or otherwise influence medical decisions. POLICY STATEMENT Gateway Health℠ does not provide coverage in the Company’s Medicaid products for breast scintimammography. The service is considered experimental and investigational in all applications, including but not limited to use as an adjunct to mammography or in staging the axillary lymph nodes. This policy is designed to address medical necessity guidelines that are appropriate for the majority of individuals with a particular disease, illness or condition. Each person’s unique clinical circumstances warrant individual consideration, based upon review of applicable medical records. (Current applicable Pennsylvania HealthChoices Agreement Section V. Program Requirements, B. Prior Authorization of Services, 1. General Prior Authorization Requirements.) Policy No. MP-105-MD-PA Page 1 of 5 DEFINITIONS Prior Authorization Review Panel – A panel of representatives from within the Pennsylvania Department of Human Services who have been assigned organizational responsibility for the review, approval and denial of all PH-MCO Prior Authorization policies and procedures. Scintimammography A noninvasive supplemental diagnostic testing technology that requires the use of radiopharmaceuticals in order to detect tissues within the breast that accumulate higher levels of radioactive tracer that emit gamma radiation. -

Procedure Guideline for Breast Scintigraphy
Procedure Guideline for Breast Scintigraphy Iraj Khalkhali, Linda E. Diggles, Raymond Taillefer, Penny R. Vandestreek, Patrick J. Peller and Hani H. Abdel-Nabi Harbor-UCLA Medical Center, Terranee; Nuclear Imaging Consultants, Roseville, California; Hospital Hôtel-Dieu de Montreal, Montreal, Quebec, Canada; Lutheran General Hospital, Park Ridge, Illinois; and University of Buffalo, Buffalo, New York Key Words: breast scintigraphy;procedureguideline should be available, as well as sonograms, if J NucÃMed 1999; 40:1233-1235 obtained. 2. A breast physical examination must be performed by either the nuclear medicine physician or the PART I: PURPOSE referring physician. 3. The time of last menses and pregnancy and lactat- The purpose of this guideline is to assist nuclear medicine ing status of the patient should be determined. practitioners in recommending, performing, interpreting and reporting the results of 99mTc-sestamibi breast scintigraphy 4. Breast scintigraphy should be delayed at least 2 wk after cyst or fine-needle aspiration, and 4—6wk (mammoscintigraphy, scintimammography). after core or excisional biopsy. 5. The nuclear medicine physician should be aware of PART II: BACKGROUND INFORMATION AND DEFINITIONS physical signs and symptoms and prior surgical procedures or therapy. Breast scintigraphy is performed after intravenous admin istration of "mTc-sestamibi and includes planar and/or C. Precautions None SPECT. D. Radiopharmaceutical 1. Intravenous injection of 740-1110 MBq (20-30 PART III: COMMON INDICATIONS AND APPLICATIONS mCi) 99mTc-sestamibi should be administered in an A. Evaluate breast cancer in patients in whom mammog- arm vein contralateral to the breast with the sus raphy is not diagnostic or is difficult to interpret (e.g., pected abnormality. -

Understanding Cost & Coverage Issues with Diagnostic Breast Imaging
Understanding Cost & Coverage Issues with Diagnostic Breast Imaging JANUARY 2019 The following report represents key findings from The Martec Group’s primary and secondary research efforts. The team was instructed to explore the cost and coverage issue with breast diagnostic imaging in order to equip Susan G. Komen with the information necessary to strategize efforts at the state and federal levels. TABLE OF CONTENTS: I. A Brief Summary of Findings ................................................................................................................... 3 II. Project Background ..................................................................................................................................... 3 III. Study Objectives ........................................................................................................................................... 3 IV. Research Methodology .............................................................................................................................. 3 V. Patient Perspective ...................................................................................................................................... 4 VI. Health/Insurance Professional Perspective ...................................................................................... 5 VII. Cost Analysis .................................................................................................................................................. 6 VIII. Study Conclusions ....................................................................................................................................... -

A Molecular Approach to Breast Imaging
Journal of Nuclear Medicine, published on January 16, 2014 as doi:10.2967/jnumed.113.126102 FOCUS ON MOLECULAR IMAGING A Molecular Approach to Breast Imaging Amy M. Fowler Department of Radiology, University of Wisconsin–Madison, Madison, Wisconsin malignant cells. A recent meta-analysis of the accuracy of 99mTc-sestamibi scintimammography as an adjunct to di- Molecular imaging is a multimodality discipline for noninvasively agnostic mammography for detection of breast cancer dem- visualizing biologic processes at the subcellular level. Clinical applications of radionuclide-based molecular imaging for breast onstrated a sensitivity of 83% and specificity of 85% (2). cancer continue to evolve. Whole-body imaging, with scinti- However, sensitivity was less for nonpalpable (59%) versus mammography and PET, and newer dedicated breast imaging palpable lesions (87%) despite comparable specificity, with systems are reviewed. The potential clinical indications and the no significant difference between planar and SPECT meth- challenges of implementing these emerging technologies are ods. Decreased sensitivity for nonpalpable, presumably presented. smaller, lesions is in part due to the limited spatial resolu- Key Words: molecular imaging; oncology; breast; PET; PET/ tion of conventional g cameras. CT; radiopharmaceuticals; breast cancer; breast-specific g im- In addition to 99mTc-sestamibi, the positron-emitting ra- aging; positron-emission mammography; positron-emission to- diopharmaceutical 18F-FDG accumulates in many types of mography cancer including breast. Meta-analyses of the accuracy of J Nucl Med 2014; 55:1–4 whole-body 18F-FDG PET used after standard diagnostic DOI: 10.2967/jnumed.113.126102 workup for patients with suspected breast lesions demon- strated sensitivities of 83%–89% and specificities of 74%– 80% (3,4). -

Breast Ultrasound Accreditation Program Requirements
Breast Ultrasound Accreditation Program Requirements OVERVIEW ........................................................................................................................................................................... 1 MANDATORY ACCREDITATION TIME REQUIREMENTS .......................................................................................................... 2 PERSONNEL QUALIFICATIONS ..................................................................................................................................... 2 INTERPRETING PHYSICIAN .................................................................................................................................................... 2 SONOGRAPHER/TECHNOLOGIST ........................................................................................................................................... 5 EQUIPMENT ......................................................................................................................................................................... 5 QUALITY CONTROL .......................................................................................................................................................... 6 ACCEPTANCE TESTING ......................................................................................................................................................... 6 ANNUAL SURVEY ................................................................................................................................................................ -

Evaluation of Nipple Discharge
New 2016 American College of Radiology ACR Appropriateness Criteria® Evaluation of Nipple Discharge Variant 1: Physiologic nipple discharge. Female of any age. Initial imaging examination. Radiologic Procedure Rating Comments RRL* Mammography diagnostic 1 See references [2,4-7]. ☢☢ Digital breast tomosynthesis diagnostic 1 See references [2,4-7]. ☢☢ US breast 1 See references [2,4-7]. O MRI breast without and with IV contrast 1 See references [2,4-7]. O MRI breast without IV contrast 1 See references [2,4-7]. O FDG-PEM 1 See references [2,4-7]. ☢☢☢☢ Sestamibi MBI 1 See references [2,4-7]. ☢☢☢ Ductography 1 See references [2,4-7]. ☢☢ Image-guided core biopsy breast 1 See references [2,4-7]. Varies Image-guided fine needle aspiration breast 1 Varies *Relative Rating Scale: 1,2,3 Usually not appropriate; 4,5,6 May be appropriate; 7,8,9 Usually appropriate Radiation Level Variant 2: Pathologic nipple discharge. Male or female 40 years of age or older. Initial imaging examination. Radiologic Procedure Rating Comments RRL* See references [3,6,8,10,13,14,16,25- Mammography diagnostic 9 29,32,34,42-44,71-73]. ☢☢ See references [3,6,8,10,13,14,16,25- Digital breast tomosynthesis diagnostic 9 29,32,34,42-44,71-73]. ☢☢ US is usually complementary to mammography. It can be an alternative to mammography if the patient had a recent US breast 9 mammogram or is pregnant. See O references [3,5,10,12,13,16,25,30,31,45- 49]. MRI breast without and with IV contrast 1 See references [3,8,23,24,35,46,51-55]. -

Screening Automated Whole Breast Ultrasound
Screening Automated Whole Breast Ultrasound Screening Automated Whole Breast Ultrasound Stanford now offers screening automated whole breast ultrasound (SAWBU) at our Stanford Medicine Cancer Center Palo Alto location. This is an optional test that can be used as a supplement to screening mammography in women with mammographically dense breasts. It can find cancers that cannot be seen on mammograms due to overlap with dense breast tissue. Stanford uses automated whole breast technique, a new method developed for accuracy and efficiency. Who is a candidate for SAWBU What will happen during the How is SAWBU exam is examination? SAWBU examination? different? This is an optional test to supplement You will lie on your back, and gel will Screening automated screening mammography in women be applied to your breast. whole breast ultrasound who: uses sound waves (no radi- A large ultrasound handpiece will be • Undergo routine screening with ation) to create 3D pictures placed on the breast, and the system mammography. of the breast tissue, using will automatically take a “sweep” • Have no current signs or a new automated method that obtains ultrasound images of symptoms of breast cancer. developed for accuracy and the tissue from top to bottom. The • Have mammographically dense efficiency. handpiece will be repositioned to take (heterogeneously or extremely other “sweeps” to include all of the It can find cancers that dense) breasts. breast tissue. cannot be seen on mam- • Are not at “high risk" undergoing mograms alone due to supplemental screening with An exam of both breasts takes less overlap with dense breast breast MRI. Screening ultra- than 20 minutes to obtain. -
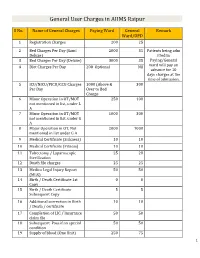
General User Charges in AIIMS Raipur
General User Charges in AIIMS Raipur S No. Name of General Charges Paying Ward General Remark Ward/OPD 1 Registration Charges 200 25 2 Bed Charges Per Day (Sami 2000 35 Patients being adm Deluxe) itted in 3 Bed Charges Per Day (Deluxe) 3000 35 Paying/General 4 Diet Charges Per Day 200 Optional Nil ward will pay an advance for 10 days charges at the time of admission. 5 ICU/NICU/PICU/CCU Charges 1000 (Above & 300 Per Day Over to Bed Charge 6 Minor Operation in OT/MOT 250 100 not mentioned in list, under L A 7 Minor Operation in OT/MOT 1000 300 not mentioned in list, under G A 8 Major Operation in OT, Not 2000 1000 mentioned in list under G A 9 Medical Certificate (Sickness) 10 10 10 Medical Certificate (Fitness) 10 10 11 Tubectomy / Laparoscopic 25 20 Sterilization 12 Death file charges 25 25 13 Medico Legal Injury Report 50 50 (MLR) 14 Birth / Death Certificate 1st 0 0 Copy 15 Birth / Death Certificate 5 5 Subsequent Copy 16 Additional correction in Birth 10 10 / Death / certificate 17 Completion of LIC / Insurance 50 50 claim file 18 Subsequent Pass if on special 50 50 condition 19 Supply of blood (One Unit) 250 75 1 20 Medical Board Certificate 500 500 On Special Case User Charges for Investigations in AIIMS Raipur S No. Name of Investigations Paying General Remark Ward Ward/OPD Anaesthsia 1 ABG 75 50 2 ABG ALONGWITH 150 100 ELECTROLYTES(NA+,K+)(Na,K) 3 ONLY ELECTROLYTES(Na+,K+,Cl,Ca+) 75 50 4 ONLY CALCIUM 50 25 5 GLUCOSE 25 20 6 LACTATE 25 20 7 UREA. -

Commercial Reimbursement and Utilization for Invenia ABUS (Automated Breast Ultrasound)
Commercial Reimbursement and Utilization for Invenia ABUS (Automated Breast Ultrasound) Background interpreting physician, referring physician/Health Care Provider (HCP), and patient.6 Specifically, the MQSA encourages education More than one-half of women younger than 50 years old and and outreach to patients from medical/scientific organizations, approximately one-third of women 50 years and older have industry, and interpreting physicians. In addition, outreach to dense breasts in the US.1 Higher breast density is associated referring physicians is encouraged from professional organizations with decreased mammographic sensitivity and specificity and and Continuing Medical Education (CME) providers.6 increased breast cancer risk.2 Due to the shortcomings of mammography in this population of women, an individualized Despite widely available options for additional screening and multimodal screening approach is recommended to improve the legislation mandating density inform, uptake and adoption of early detection of breast cancer. No clinical guidelines explicitly supplemental screening has lagged. In addition to knowledge recommend use of supplemental breast cancer screening on gaps, one study identified reimbursement uncertainties (e.g. women with dense breasts,3 but as of March 2018, 33 states have billing, coding and coverage) as a key factor for slow uptake. In enacted legislation requiring that breast density, in addition to 2015, the CPT® codebook was updated to include two new codes mammography results, be reported to women undergoing such for breast ultrasound, CPT 76641 and 76642, for complete and procedures; additional states have introduced or are currently limited exams, respectively.7 These codes replaced CPT 76645. working on legislation.4 Most states require specific density The new and old codes alike do not differentiate the technology inform language distinguishing dense (i.e., BI-RADS® C and D) used to perform an exam (handheld vs. -

Breast Imaging Faqs
Breast Imaging Frequently Asked Questions Update 2021 The following Q&As address Medicare guidelines on the reporting of breast imaging procedures. Private payer guidelines may vary from Medicare guidelines and from payer to payer; therefore, please be sure to check with your private payers on their specific breast imaging guidelines. Q: What differentiates a diagnostic from a screening mammography procedure? Medicare’s definitions of screening and diagnostic mammography, as noted in the Centers for Medicare and Medicaid’s (CMS’) National Coverage Determination database, and the American College of Radiology’s (ACR’s) definitions, as stated in the ACR Practice Parameter of Screening and Diagnostic Mammography, are provided as a means of differentiating diagnostic from screening mammography procedures. Although Medicare’s definitions are consistent with those from the ACR, the ACR's definitions of screening and diagnostic mammography offer additional insight into what may be included in these procedures. Please go to the CMS and ACR Web site links noted below for more in- depth information about these studies. Medicare Definitions (per the CMS National Coverage Determination for Mammograms 220.4) “A diagnostic mammogram is a radiologic procedure furnished to a man or woman with signs and symptoms of breast disease, or a personal history of breast cancer, or a personal history of biopsy - proven benign breast disease, and includes a physician's interpretation of the results of the procedure.” “A screening mammogram is a radiologic procedure furnished to a woman without signs or symptoms of breast disease, for the purpose of early detection of breast cancer, and includes a physician’s interpretation of the results of the procedure.