Breast Scintimammography
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

BREAST IMAGING for SCREENING and DIAGNOSING CANCER Policy Number: DIAGNOSTIC 105.9 T2 Effective Date: January 1, 2017
Oxford UnitedHealthcare® Oxford Clinical Policy BREAST IMAGING FOR SCREENING AND DIAGNOSING CANCER Policy Number: DIAGNOSTIC 105.9 T2 Effective Date: January 1, 2017 Table of Contents Page Related Policies INSTRUCTIONS FOR USE .......................................... 1 Omnibus Codes CONDITIONS OF COVERAGE ...................................... 1 Preventive Care Services BENEFIT CONSIDERATIONS ...................................... 2 Radiology Procedures Requiring Precertification for COVERAGE RATIONALE ............................................. 3 eviCore Healthcare Arrangement APPLICABLE CODES ................................................. 5 DESCRIPTION OF SERVICES ...................................... 6 CLINICAL EVIDENCE ................................................. 7 U.S. FOOD AND DRUG ADMINISTRATION ................... 16 REFERENCES .......................................................... 18 POLICY HISTORY/REVISION INFORMATION ................ 22 INSTRUCTIONS FOR USE This Clinical Policy provides assistance in interpreting Oxford benefit plans. Unless otherwise stated, Oxford policies do not apply to Medicare Advantage members. Oxford reserves the right, in its sole discretion, to modify its policies as necessary. This Clinical Policy is provided for informational purposes. It does not constitute medical advice. The term Oxford includes Oxford Health Plans, LLC and all of its subsidiaries as appropriate for these policies. When deciding coverage, the member specific benefit plan document must be referenced. The terms -

EARLY DETECTION Breast Health Awareness and Clinical Breast Exam
EARLY DETECTION Breast Health Awareness and Clinical Breast Exam Knowledge Summary EARLY DETECTION Breast Health Awareness and Clinical Breast Exam INTRODUCTION KEY SUMMARY Early diagnosis of breast cancer begins with the establish- Early detection programs ment of programs to improve early detection of symptomatic ¬ Early diagnosis of breast cancer can improve survival, lower women, or women with breast lumps that patients and their morbidity and reduces the cost of care when followed by a providers can feel. Early recognition of symptoms and accu- prompt diagnosis and effective treatment. rate diagnosis of breast cancer can result in cancers being diagnosed at earlier stages when treatment is more feasible, ¬ An effective early diagnosis program includes: affordable and effective. This requires that health systems √ Breast health awareness education. have trained frontline personnel who are able to recognize the √ Reducing barriers to accessing care. signs and symptoms of breast abnormalities for both benign √ Clinical breast exam (CBE) performed by primary care breast issues as well as cancers, perform clinical breast exam providers. (CBE) and know the proper referral protocol when diagnostic √ Timely diagnosis for all women found to have abnormal workup is warranted. Women who can identify breast abnor- findings and timely treatment for all women proven by malities, who have timely access to health clinical evaluation, tissue diagnosis to have breast cancer. diagnosis and treatment and who are empowered to seek this √ If supported by evidence, a quality screening mammogra- care are more likely to be diagnosed at an earlier stage (see phy program performed in a cost-effective, resource-sus- Planning: Improving Access to Breast Cancer Care). -

Breast Imaging H
BREAST IMAGING H. Lee Moffitt Cancer Center and Research Institute Rotation Director: Margaret Szabunio, M.D. General Goals : On this rotation, the resident will learn to interpret screening mammograms and to perform diagnostic mammography and ultrasound examinations of the breast. The resident will learn to formulate appropriate differential diagnoses and recommendations for various breast pathologies. The resident will also learn mammographic, ultrasound and MR breast biopsy techniques. Daily Work : The resident rotation begins after morning conference has concluded. In this rotation the resident shall learn BIRADS nomenclature and become proficient in using the PENRAD system for reporting. The resident will also learn the difference between screening and diagnostic mammography and how to perform a diagnostic work-up. (S)he will become familiarized with mammographic positioning and technique and quality assurance including MQSA and ACR requirements. The resident will learn to interpret mammographic images and the use of additional mammographic views for problem solving. (S)he will learn when and how to employ sonography in patient evaluation. The resident is REQUIRED to attend Thursday morning breast interdisciplinary conference. Preparing and reviewing cases for this conference is highly recommended. The resident will assist with and perform needle localizations, breast biopsy and cyst aspiration procedures using mammographic, stereotactic and sonographic techniques for each. The resident is expected to identify proper indications and contraindications for each procedure and how to identify and manage complications. The resident is expected to understand and complete informed consent for image guided breast procedures. On occasion, the resident may observe or assist with ductography procedures. Opportunity to observe and assist with MR guided breast procedures may also be available. -

Procedure Guideline for Breast Scintigraphy
Procedure Guideline for Breast Scintigraphy Iraj Khalkhali, Linda E. Diggles, Raymond Taillefer, Penny R. Vandestreek, Patrick J. Peller and Hani H. Abdel-Nabi Harbor-UCLA Medical Center, Terranee; Nuclear Imaging Consultants, Roseville, California; Hospital Hôtel-Dieu de Montreal, Montreal, Quebec, Canada; Lutheran General Hospital, Park Ridge, Illinois; and University of Buffalo, Buffalo, New York Key Words: breast scintigraphy;procedureguideline should be available, as well as sonograms, if J NucÃMed 1999; 40:1233-1235 obtained. 2. A breast physical examination must be performed by either the nuclear medicine physician or the PART I: PURPOSE referring physician. 3. The time of last menses and pregnancy and lactat- The purpose of this guideline is to assist nuclear medicine ing status of the patient should be determined. practitioners in recommending, performing, interpreting and reporting the results of 99mTc-sestamibi breast scintigraphy 4. Breast scintigraphy should be delayed at least 2 wk after cyst or fine-needle aspiration, and 4—6wk (mammoscintigraphy, scintimammography). after core or excisional biopsy. 5. The nuclear medicine physician should be aware of PART II: BACKGROUND INFORMATION AND DEFINITIONS physical signs and symptoms and prior surgical procedures or therapy. Breast scintigraphy is performed after intravenous admin istration of "mTc-sestamibi and includes planar and/or C. Precautions None SPECT. D. Radiopharmaceutical 1. Intravenous injection of 740-1110 MBq (20-30 PART III: COMMON INDICATIONS AND APPLICATIONS mCi) 99mTc-sestamibi should be administered in an A. Evaluate breast cancer in patients in whom mammog- arm vein contralateral to the breast with the sus raphy is not diagnostic or is difficult to interpret (e.g., pected abnormality. -

A Molecular Approach to Breast Imaging
Journal of Nuclear Medicine, published on January 16, 2014 as doi:10.2967/jnumed.113.126102 FOCUS ON MOLECULAR IMAGING A Molecular Approach to Breast Imaging Amy M. Fowler Department of Radiology, University of Wisconsin–Madison, Madison, Wisconsin malignant cells. A recent meta-analysis of the accuracy of 99mTc-sestamibi scintimammography as an adjunct to di- Molecular imaging is a multimodality discipline for noninvasively agnostic mammography for detection of breast cancer dem- visualizing biologic processes at the subcellular level. Clinical applications of radionuclide-based molecular imaging for breast onstrated a sensitivity of 83% and specificity of 85% (2). cancer continue to evolve. Whole-body imaging, with scinti- However, sensitivity was less for nonpalpable (59%) versus mammography and PET, and newer dedicated breast imaging palpable lesions (87%) despite comparable specificity, with systems are reviewed. The potential clinical indications and the no significant difference between planar and SPECT meth- challenges of implementing these emerging technologies are ods. Decreased sensitivity for nonpalpable, presumably presented. smaller, lesions is in part due to the limited spatial resolu- Key Words: molecular imaging; oncology; breast; PET; PET/ tion of conventional g cameras. CT; radiopharmaceuticals; breast cancer; breast-specific g im- In addition to 99mTc-sestamibi, the positron-emitting ra- aging; positron-emission mammography; positron-emission to- diopharmaceutical 18F-FDG accumulates in many types of mography cancer including breast. Meta-analyses of the accuracy of J Nucl Med 2014; 55:1–4 whole-body 18F-FDG PET used after standard diagnostic DOI: 10.2967/jnumed.113.126102 workup for patients with suspected breast lesions demon- strated sensitivities of 83%–89% and specificities of 74%– 80% (3,4). -

Biopsies of the Breast
American Cancer Society After the procedure is complete, pressure will be applied to the needle site to help stop any bleeding and a bandage will be applied (usually an adhesive Guidelines strip). The procedure takes approximately 30 minutes. Regarding Breast Health Core Needle • Breast Self-Exam (BSE) – More recently the Your Results focus of BSE has been moving from the monthly Your specimens will be delivered to a pathologist who routine self-exam to becoming more self-aware Biopsy will examine them under a microscope. The findings of your breast changes and seeking help if any will be reported to your healthcare provider who will, in abnormalities are noticed. BSE represents a turn, forward the results on to you. structured way in which the breasts can be examined effectively. You should know how your Your Questions breasts normally feel and look. We realize this is a stressful time for you. As our patient, Beginning in their 20’s, women should learn the we want you to be as confident and informed about benefits of BSE. You can be instructed on the your healthcare as you can be. We hope this brochure proper techniques of BSE at the time of your has been informative for you. Please feel free to ask us routine health examination. You should also know any questions you may have. that there are limitations to BSE. Report any breast changes that you notice to your healthcare provider immediately. • Clinical Breast Exam – Women between the Risks ages of 20 and 30 should have a breast exam by a • There is a slight chance of developing bleeding healthcare provider every three years. -

Evaluation of Nipple Discharge
New 2016 American College of Radiology ACR Appropriateness Criteria® Evaluation of Nipple Discharge Variant 1: Physiologic nipple discharge. Female of any age. Initial imaging examination. Radiologic Procedure Rating Comments RRL* Mammography diagnostic 1 See references [2,4-7]. ☢☢ Digital breast tomosynthesis diagnostic 1 See references [2,4-7]. ☢☢ US breast 1 See references [2,4-7]. O MRI breast without and with IV contrast 1 See references [2,4-7]. O MRI breast without IV contrast 1 See references [2,4-7]. O FDG-PEM 1 See references [2,4-7]. ☢☢☢☢ Sestamibi MBI 1 See references [2,4-7]. ☢☢☢ Ductography 1 See references [2,4-7]. ☢☢ Image-guided core biopsy breast 1 See references [2,4-7]. Varies Image-guided fine needle aspiration breast 1 Varies *Relative Rating Scale: 1,2,3 Usually not appropriate; 4,5,6 May be appropriate; 7,8,9 Usually appropriate Radiation Level Variant 2: Pathologic nipple discharge. Male or female 40 years of age or older. Initial imaging examination. Radiologic Procedure Rating Comments RRL* See references [3,6,8,10,13,14,16,25- Mammography diagnostic 9 29,32,34,42-44,71-73]. ☢☢ See references [3,6,8,10,13,14,16,25- Digital breast tomosynthesis diagnostic 9 29,32,34,42-44,71-73]. ☢☢ US is usually complementary to mammography. It can be an alternative to mammography if the patient had a recent US breast 9 mammogram or is pregnant. See O references [3,5,10,12,13,16,25,30,31,45- 49]. MRI breast without and with IV contrast 1 See references [3,8,23,24,35,46,51-55]. -

Advanced Breast Imaging: MBI, ABUS, Tomo, CE-MRI, Fast-MRI, Breast PEM
Advanced Breast Imaging: MBI, ABUS, Tomo, CE-MRI, Fast-MRI, Breast PEM Thursday, May 2, 2019 7:00 AM-12:00 PM COURSE MODERATORS: Paul Baron, MD and Souzan El-Eid, MD FACULTY: Paul Baron, MD; Souzan El-Eid, MD; Ian Grady, MD; Alan Hollingsworth, MD; Jessica Leung, MD; Jocelyn Rapelyea, MD COURSE DESCRIPTION: Advances in breast imaging have been creating as much controversy as improvements in patient care. Is screening mammography still the standard of care for breast cancer screening? Should high-risk patients and/or patients with dense breasts undergo annual gadolinium-enhanced MRI, and how does “fast” MRI impact the risk, benefit, and cost balance? If you could acquire or utilize only one supplemental imaging modality, should you use whole-breast ultrasound (manual or automated), breast MRI, molecular breast imaging, or breast positron emission mammography, and what are the pros and cons of each? This course will focus on bringing the surgeon up to speed on current and emerging breast imaging modalities; screening for average-risk versus high-risk patients, with discussions of how various technologies work, the supporting data, and the economic implications. COURSE OBJECTIVES: At the conclusion of this course, participants should be able to: • Identify current treatment and management options for breast cancer patients • Discuss the pros and cons of available screening modalities • Determine the optimal screening regimen for average-risk and high-risk patients This course will offer participants an opportunity to become familiar with -

Evaluation of the Quantitative Accuracy of a Commercially-Available Positron Emission Mammography Scanner
The Texas Medical Center Library DigitalCommons@TMC The University of Texas MD Anderson Cancer Center UTHealth Graduate School of The University of Texas MD Anderson Cancer Biomedical Sciences Dissertations and Theses Center UTHealth Graduate School of (Open Access) Biomedical Sciences 8-2010 EVALUATION OF THE QUANTITATIVE ACCURACY OF A COMMERCIALLY-AVAILABLE POSITRON EMISSION MAMMOGRAPHY SCANNER Adam Springer Follow this and additional works at: https://digitalcommons.library.tmc.edu/utgsbs_dissertations Part of the Diagnosis Commons, Equipment and Supplies Commons, and the Other Medical Sciences Commons Recommended Citation Springer, Adam, "EVALUATION OF THE QUANTITATIVE ACCURACY OF A COMMERCIALLY-AVAILABLE POSITRON EMISSION MAMMOGRAPHY SCANNER" (2010). The University of Texas MD Anderson Cancer Center UTHealth Graduate School of Biomedical Sciences Dissertations and Theses (Open Access). 64. https://digitalcommons.library.tmc.edu/utgsbs_dissertations/64 This Thesis (MS) is brought to you for free and open access by the The University of Texas MD Anderson Cancer Center UTHealth Graduate School of Biomedical Sciences at DigitalCommons@TMC. It has been accepted for inclusion in The University of Texas MD Anderson Cancer Center UTHealth Graduate School of Biomedical Sciences Dissertations and Theses (Open Access) by an authorized administrator of DigitalCommons@TMC. For more information, please contact [email protected]. EVALUATION OF THE QUANTITATIVE ACCURACY OF A COMMERCIALLY- AVAILABLE POSITRON EMISSION MAMMOGRAPHY SCANNER -

Clinical Guidelines for the Management of Breast Cancer West Midlands Expert Advisory Group for Breast Cancer West Midlands Clinical Networks and Clinical Senate
Clinical Guidelines for the Management of Breast Cancer West Midlands Expert Advisory Group for Breast Cancer West Midlands Clinical Networks and Clinical Senate Coversheet for Network Expert Advisory Group Agreed Documentation This sheet is to accompany all documentation agreed by the West Midlands Strategic Clinical Network Expert Advisory Groups. This will assist the Clinical Network to endorse the documentation and request implementation. EAG name Breast Cancer Expert Advisory Group Document Clinical guidelines for the management of breast cancer Title Published December 2016 date Document Clinical guidance for the management of Breast cancer to all practitioners, Purpose clinicians and health care professionals providing a service to all patients across the West Midlands Clinical Network. Authors Original Author: Mr Stephen Parker Modified By: Mrs Abigail Tomlins Consultant Breast Surgeon University Hospitals Coventry & Warwickshire NHS Trust References Consultation These guidelines were originally authored by Stephen Parker and Process subsequently modified by Abigail Tomlins for the Coventry, Warwickshire and Worcestershire Breast Group. The West Midlands EAG agreed to adopt these guidelines as the regional network guidelines. The version history reflects changes made by the Coventry, Warwickshire and Worcestershire Breast Group. As the Coventry, Warwickshire and Worcestershire Breast Group update their guidelines, the EAG will discuss whether to adopt the updated version. Review Date December 2019 (must be within three years) Approval Network Clinical Director Signatures: Date: 25/10/2017 \\ims.gov.uk\data\Users\GBEXPVD\EXPHOME25\PGoulding\Data\Desktop\guidelines- 2 for-the-management-of-breast-cancer-v1.doc Version History - Coventry, Warwickshire and Worcestershire Breast Group Version Date Brief Summary of Change 2010v1.0D 12 March 2010 Immediate breast reconstruction criteria Young adult survivors Updated follow-up guidelines. -
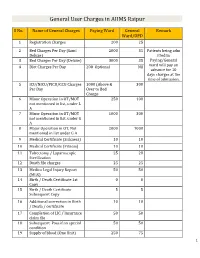
General User Charges in AIIMS Raipur
General User Charges in AIIMS Raipur S No. Name of General Charges Paying Ward General Remark Ward/OPD 1 Registration Charges 200 25 2 Bed Charges Per Day (Sami 2000 35 Patients being adm Deluxe) itted in 3 Bed Charges Per Day (Deluxe) 3000 35 Paying/General 4 Diet Charges Per Day 200 Optional Nil ward will pay an advance for 10 days charges at the time of admission. 5 ICU/NICU/PICU/CCU Charges 1000 (Above & 300 Per Day Over to Bed Charge 6 Minor Operation in OT/MOT 250 100 not mentioned in list, under L A 7 Minor Operation in OT/MOT 1000 300 not mentioned in list, under G A 8 Major Operation in OT, Not 2000 1000 mentioned in list under G A 9 Medical Certificate (Sickness) 10 10 10 Medical Certificate (Fitness) 10 10 11 Tubectomy / Laparoscopic 25 20 Sterilization 12 Death file charges 25 25 13 Medico Legal Injury Report 50 50 (MLR) 14 Birth / Death Certificate 1st 0 0 Copy 15 Birth / Death Certificate 5 5 Subsequent Copy 16 Additional correction in Birth 10 10 / Death / certificate 17 Completion of LIC / Insurance 50 50 claim file 18 Subsequent Pass if on special 50 50 condition 19 Supply of blood (One Unit) 250 75 1 20 Medical Board Certificate 500 500 On Special Case User Charges for Investigations in AIIMS Raipur S No. Name of Investigations Paying General Remark Ward Ward/OPD Anaesthsia 1 ABG 75 50 2 ABG ALONGWITH 150 100 ELECTROLYTES(NA+,K+)(Na,K) 3 ONLY ELECTROLYTES(Na+,K+,Cl,Ca+) 75 50 4 ONLY CALCIUM 50 25 5 GLUCOSE 25 20 6 LACTATE 25 20 7 UREA. -

Consensus Guideline on Concordance Assessment of Image-Guided Breast Biopsies and Management of Borderline Or High-Risk Lesions
- Official Statement - Consensus Guideline on Concordance Assessment of Image-Guided Breast Biopsies and Management of Borderline or High-Risk Lesions Purpose To outline the management approach for borderline and high risk lesions identified on image-guided breast biopsy. Associated ASBrS Guidelines or Quality Measures 1. Image-Guided Percutaneous Biopsy of Palpable and Nonpalpable Breast Lesions 2. Performance and Practice Guidelines for Stereotactic Breast Procedures 3. Concordance Assessment Following Image-Guided Breast Biopsy Methods Literature review inclusive of recent randomized controlled trials evaluating the management of various borderline and high-risk lesions (including atypical hyperplasia, lobular neoplasia, papillary lesions, radial scars and complex sclerosing lesions, fibroepithelial lesions, mucocele-like lesions, spindle cell lesions, and pseudoangiomatous stromal hyperplasia [PASH]) identified on image-guided breast biopsies. This is not a complete systematic review but a comprehensive review of the modern literature on this subject. The ASBS Research Committee developed a consensus document which the ASBS Board of Directors reviewed and approved. Summary of Data Reviewed Percutaneous core needle biopsy (CNB) is the preferred, initial, minimally invasive diagnostic procedure for nonpalpable breast lesions or palpable breast masses.1 Concordance assessment of the histologic, imaging, and clinical findings determines further management. Discordance refers to the situation in which a breast CNB demonstrates benign histology, while the clinical or imaging findings are suspicious for malignancy. If there is discordance between imaging and pathology, histological evaluation is still needed. This can be accomplished either by repeat CNB, perhaps with consideration of larger gauge or vacuum- assisted device, or surgical excision.2-5 Some nonmalignant CNB findings are considered “borderline” because of their potential association with malignancy.