Functional and Evolutionary Relationships Between Terpene Synthases from Australian Myrtaceae
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Pinturas Antiincrustantes Derivadas De Plantas Terrestres: Una Solución Segura Para El Ambiente En El Control De La Bioincrustación
ISSN 1688-6593 INNOTEC 2021, No. 22 (e559) https://doi.org/10.26461/22.01 REVISTA DEL LABORATORIO TECNOLÓGICO DEL URUGUAY Revisiones Pinturas antiincrustantes derivadas de plantas terrestres: una solución segura para el ambiente en el control de la bioincrustación Antifouling paints derived from terrestrial plants: a safe solution for the environment in the control of biofouling Tintas anti-incrustantes derivadas de plantas terrestres: uma solução segura para o meio ambiente no controle da bioincrustação Vanessa Ochi Agostini 1,* https://orcid.org/0000-0002-8325-254X Grasiela Lopes Leães Pinho1 https://orcid.org/0000-0001-7951-0334 Erik Muxagata2 http://orcid.org/0000-0002-4210-5252 Alexandre José Macedo3 https://orcid.org/0000-0002-8951-4029 Fabiana Rey Bentos4 https://orcid.org/0000-0001-5931-5902 Lucía Boccardi4 https://orcid.org/0000-0002-5391-2308 María Jesús Dabezies5 https://orcid.org/0000-0001-9909-3427 Ernesto Brugnoli6 https://orcid.org/0000-0001-7304-1856 *Autor de contacto: [email protected] 1 Laboratório de Microcontaminantes Orgânicos e Ecotoxicologia Aquática – Programa de Pós-Graduação em Oceanologia – Instituto de Oceanografia – Universidade Federal do Rio Grande, Rio Grande do Sul, Brasil 2 Laboratório de Zooplâncton – Programa de Pós-Graduação em Oceanografia Biológica – Instituto de Oceanografia – Universidade Federal do Rio Grande, Rio Grande do Sul, Brasil 3 Laboratório de Biofilmes e Diversidade Bacteriana – Centro de Biotecnologia – Universidade Federal do Rio Grande do Sul, Rio Grande do Sul, Brasil 4 Latitud–Fundación LATU, Montevideo, Uruguay 5 Laboratorio Tecnológico del Uruguay (LATU), Montevideo, Uruguay 6 Oceanografía y Ecología Marina, Facultad de Ciencias, Universidad de la República, Montevideo, Uruguay Recepción: 14 Agosto 2020 Aprobación: 15 Enero 2021 Esta obra está bajo una Licencia Creative Commons Atribución-NoComercial 4.0 Internacional. -

Eucalyptus Rubida Subsp. Rubida
Plants of South Eastern New South Wales Flower buds on leafy stem. Australian Plant Image Index, photographer Peter Ormay, Canberra, ACT Juvenile leaves. Australian Plant Image Index, photographer Peter Ormay, Canberra, ACT Line drawings. e. juvenile (right) and adult (left) Tree. Photographer Jackie Miles leaves; buds and gumnuts. KR Thiele, Australian National Herbarium, © 2021 Royal Botanic Gardens Board, Melbourne, Vic Common name Candlebark, Ribbon gum, White gum Family Myrtaceae Where found Dry forest, woodland, and grassy areas, usually on cold flats. Western Slopes, Kosciuszko National Park, the mountains to the north, ACT, and tablelands. Occasionally in the ranges. Notes Tree to 40 m tall. Bark smooth throughout or with some patches of rough, greyish bark at the base. Rough bark shortly fibrous, platy, at times shedding irregularly, grey to grey-black. Smooth bark shedding in long ribbons, with horizontal black scars, often powdery. Branchlets glaucous or non-glaucous. Juvenile stems rounded in cross section, glaucous. Juvenile leaves opposite each other for many pairs, stalkless, 2-6 cm long, 25-65 mm wide, glaucous. Adult leaves alternating up the stems, 6.8-17.5 cm long, 8-34 mm wide, glossy or dull, green, grey-green, or glaucous. Flowers white, with 0 petals. Flower clusters 3- or 7- flowered. Mature flower buds 4–9 mm long, caps as long as or shorter than the base. Flowers Spring-Autumn. Gumnuts 4-8 mm in diameter. Gumnuts that have dropped their seed have protruding valves or valves that are not very noticeable. Hybridises with Eucalyptus aggregata and Eucalyptus nortonii. May hybridise with Eucalyptus parvula. -

Newsletter No
Newsletter No. 167 June 2016 Price: $5.00 AUSTRALASIAN SYSTEMATIC BOTANY SOCIETY INCORPORATED Council President Vice President Darren Crayn Daniel Murphy Australian Tropical Herbarium (ATH) Royal Botanic Gardens Victoria James Cook University, Cairns Campus Birdwood Avenue PO Box 6811, Cairns Qld 4870 Melbourne, Vic. 3004 Australia Australia Tel: (+61)/(0)7 4232 1859 Tel: (+61)/(0) 3 9252 2377 Email: [email protected] Email: [email protected] Secretary Treasurer Leon Perrie John Clarkson Museum of New Zealand Te Papa Tongarewa Queensland Parks and Wildlife Service PO Box 467, Wellington 6011 PO Box 975, Atherton Qld 4883 New Zealand Australia Tel: (+64)/(0) 4 381 7261 Tel: (+61)/(0) 7 4091 8170 Email: [email protected] Mobile: (+61)/(0) 437 732 487 Councillor Email: [email protected] Jennifer Tate Councillor Institute of Fundamental Sciences Mike Bayly Massey University School of Botany Private Bag 11222, Palmerston North 4442 University of Melbourne, Vic. 3010 New Zealand Australia Tel: (+64)/(0) 6 356- 099 ext. 84718 Tel: (+61)/(0) 3 8344 5055 Email: [email protected] Email: [email protected] Other constitutional bodies Hansjörg Eichler Research Committee Affiliate Society David Glenny Papua New Guinea Botanical Society Sarah Matthews Heidi Meudt Advisory Standing Committees Joanne Birch Financial Katharina Schulte Patrick Brownsey Murray Henwood David Cantrill Chair: Dan Murphy, Vice President Bob Hill Grant application closing dates Ad hoc adviser to Committee: Bruce Evans Hansjörg Eichler Research -

The Natural Distribution of Eucalyptus Species in Tasmania
The natural distribution of Eucalyptus species in Tasmania K.J. Williams and B.M. Potts Cooperative Research Centre for Temperate Hardwood Forestry, Department of Plant Science, University of Tasmania, GPO Box 252–55, Hobart 7001 email: [email protected]./[email protected] Abstract dispersed (E. cordata) or disjunct (E. archeri) occurrences. Most species that are rare in A summary is provided of the natural geographic Tasmania are endemics, with the exception of distributions of the 29 Tasmanian Eucalyptus E. perriniana and E. aff. radiata, although species. The work is based on over 60 000 the taxonomic status of the latter requires observations from numerous data sources. A map investigation. Unresolved issues relating to the on a 10 km x 10 km grid-cell scale is presented for natural distribution and taxonomic affinities of each species and is accompanied by graphs of the the Tasmanian eucalypt species are summarised. altitudinal range and flowering times, as well as descriptive notes on distribution and ecology, supplemented with a list of key references. The Introduction geographic pattern of species richness is examined at generic, subgeneric and series levels. Total In Tasmania and the Bass Strait islands, species richness is greater in the drier, eastern 29 native eucalypt species (one of which has regions compared to the wet, western regions of two subspecies) are recognised by Buchanan Tasmania, with highest concentrations of species (1995), from two informal subgenera, occurring mainly in the central east coast and Monocalyptus and Symphyomyrtus (Pryor and south-eastern regions. Monocalyptus species Johnson 1971). -

Critical Revision of the Genus Eucalyptus Volume 3: Parts 21-30
Critical revision of the genus eucalyptus Volume 3: Parts 21-30 Maiden, J. H. (Joseph Henry) (1859-1925) University of Sydney Library Sydney 2002 http://setis.library.usyd.edu.au/oztexts © University of Sydney Library. The texts and images are not to be used for commercial purposes without permission Source Text: Prepared from the print edition of Parts 21-30 Critical revision of the genus eucalyptus, published by William Applegate Gullick Sydney 1917. 223pp. All quotation marks are retained as data. First Published: 1917 583.42 Australian Etext Collections at botany prose nonfiction 1910-1939 Critical revision of the genus eucalyptus volume 3 (Government Botanist of New South Wales and Director of the Botanic Gardens, Sydney) “Ages are spent in collecting materials, ages more in separating and combining them. Even when a system has been formed, there is still something to add, to alter, or to reject. Every generation enjoys the use of a vast hoard bequeathed to it by antiquity, and transmits that hoard, augmented by fresh acquisitions, to future ages. In these pursuits, therefore, the first speculators lie under great disadvantages, and, even when they fail, are entitled to praise.” Macaulay's “Essay on Milton” Sydney William Applegate Gullick, Government Printer 1917 Part 21 CXIII. E. cinerea F.v.M. In Bentham's Flora Australiensis iii, 239 (1866). FOLLOWING is the original description:— A moderate-sized tree, with a whitish-brown persistent bark, somewhat fibrous, the foliage more or less glaucous or mealy white. Leaves opposite, sessile, cordate ovate or ovate-lanceolate, obtuse or acute, mostly 2 to 4 inches long (or narrow lanceolate, which are alternate and much longer.—J.H.M.). -

Patterns of Hybridization and Asymmetrical Gene Flow in Hybrid
Heredity (2010), 1–13 & 2010 Macmillan Publishers Limited All rights reserved 0018-067X/10 $32.00 www.nature.com/hdy ORIGINAL ARTICLE Patterns of hybridization and asymmetrical gene flow in hybrid zones of the rare Eucalyptus aggregata and common E. rubida DL Field1, DJ Ayre2, RJ Whelan2 and AG Young3 1Department of Ecology and Evolutionary Biology, University of Toronto, Toronto, Ontario, Canada; 2Institute for Conservation Biology, School of Biological Sciences, University of Wollongong, Wollongong, New South Wales, Australia and 3CSIRO Plant Industry, Canberra, Australian Capital Territory, Australia The patterns of hybridization and asymmetrical gene flow of long-term migration rates also indicate asymmetric gene among species are important for understanding the pro- flow, with higher migration rates from E. aggregata to hybrids cesses that maintain distinct species. We examined the compared with E. rubida. Taken together, these results potential for asymmetrical gene flow in sympatric populations indicate a greater genetic input from E. aggregata into the of Eucalyptus aggregata and Eucalyptus rubida, both long- hybrid populations. This asymmetry probably reflects differ- lived trees of southern Australia. A total of 421 adults from ences in style lengths (E. rubida: B7 mm, E. aggregata: three hybrid zones were genotyped with six microsatellite B4 mm), which can prevent pollen tubes of smaller-flowered markers. We used genealogical assignments, admixture species from fertilizing larger-flowered species. However, analysis and analyses of spatial genetic structure and spatial analyses of fine-scale genetic structure suggest that localized distribution of individuals, to assess patterns of interspecific seed dispersal (o40 m) and greater clustering between gene flow within populations. -
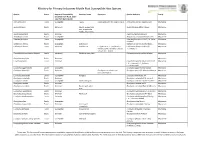
Myrtle Rust Susceptible Host Species
Ministry for Primary Industries Myrtle Rust Susceptible Host Species Species Status Degree of Susceptibility Common Name Synonyms Species Authority Family (modified from Hood, 2016 - data from Queensland) Acca sellowiana Exotic Susceptible Feijoa Feijoa sellowiana (O. Berg) O. Berg Acca sellowiana (O. Berg) Burrett Myrtaceae Agonis flexuosa Exotic Extremely Agonis, peppermint Agonis flexuosa (Willd.) Sweet Myrtaceae tree, peppermint myrtle, river myrtle Agonis juniperiana Exotic Unknown Agonis juniperiana Schaeur Myrtaceae Angophora costata Exotic Susceptible Angophora costata (Gaertn) Britten Myrtaceae Astartea fasicularis Exotic Susceptible Astartea fascicularis (Labill.) DC. (false Myrtaceae Baeckea) Callistemon citrinus Exotic Susceptible bottlebrush Callistemon citrinus (Curtis) Skeels Myrtaceae Callistemon linearis Exotic Unknown bottlebrush C. rigidus R.Br., C. pinifolius (J.C. Callistemon linearis (Schrad. & Myrtaceae Wendl.) Sweet, Melaleuca linearis J.C.Wendl.) DC Schrad. & J.C.Wendl. Chamelaucium uncinatum Schauer Exotic Extremely Geraldton wax plant Chamelaucium uncinatum Schauer Myrtaceae Corymbia calophylla Exotic Unknown Myrtaceae Corymbia ficifolia Exotic Unknown Corymbia ficifolia (F.Muell.) K.D.Hill & Myrtaceae L.A.S.Johnson (LS C. ficifolia × C. ptychocarpa) Eucalyptus agglomerata Exotic Susceptible Eucalyptus agglomerata Maiden Myrtaceae Eucalyptus bancroftii Exotic Unknown Eucalyptus tereticornis var. Eucalyptus bancroftii (Maiden) Maiden Myrtaceae bancroftii Maiden Eucalyptus botryoides Exotic Susceptible bangalay -

D.Nicolle, Classification of the Eucalypts (Angophora, Corymbia and Eucalyptus) | 2
Taxonomy Genus (common name, if any) Subgenus (common name, if any) Section (common name, if any) Series (common name, if any) Subseries (common name, if any) Species (common name, if any) Subspecies (common name, if any) ? = Dubious or poorly-understood taxon requiring further investigation [ ] = Hybrid or intergrade taxon (only recently-described and well-known hybrid names are listed) ms = Unpublished manuscript name Natural distribution (states listed in order from most to least common) WA Western Australia NT Northern Territory SA South Australia Qld Queensland NSW New South Wales Vic Victoria Tas Tasmania PNG Papua New Guinea (including New Britain) Indo Indonesia TL Timor-Leste Phil Philippines ? = Dubious or unverified records Research O Observed in the wild by D.Nicolle. C Herbarium specimens Collected in wild by D.Nicolle. G(#) Growing at Currency Creek Arboretum (number of different populations grown). G(#)m Reproductively mature at Currency Creek Arboretum. – (#) Has been grown at CCA, but the taxon is no longer alive. – (#)m At least one population has been grown to maturity at CCA, but the taxon is no longer alive. Synonyms (commonly-known and recently-named synonyms only) Taxon name ? = Indicates possible synonym/dubious taxon D.Nicolle, Classification of the eucalypts (Angophora, Corymbia and Eucalyptus) | 2 Angophora (apples) E. subg. Angophora ser. ‘Costatitae’ ms (smooth-barked apples) A. subser. Costatitae, E. ser. Costatitae Angophora costata subsp. euryphylla (Wollemi apple) NSW O C G(2)m A. euryphylla, E. euryphylla subsp. costata (smooth-barked apple, rusty gum) NSW,Qld O C G(2)m E. apocynifolia Angophora leiocarpa (smooth-barked apple) Qld,NSW O C G(1) A. -

The Vegetation Communities Dry Eucalypt Forest and Woodland
Edition 2 From Forest to Fjaeldmark The Vegetation Communities Dry eucalypt forest and woodland Eucalyptus amygdalina Edition 2 From Forest to Fjaeldmark 1 Dry eucalypt forest and woodland Community (Code) Page Eucalyptus amygdalina coastal forest and woodland (DAC) 11 Eucalyptus amygdalina forest and woodland on dolerite (DAD) 13 Eucalyptus amygdalina forest and woodland on sandstone (DAS) 15 Eucalyptus amygdalina forest on mudstone (DAM) 17 Eucalyptus amygdalina inland forest and woodland on Cainozoic deposits (DAZ) 19 Eucalyptus amygdalina–Eucalyptus obliqua damp sclerophyll forest (DSC) 22 Eucalyptus barberi forest and woodland (DBA) 24 Eucalyptus coccifera forest and woodland (DCO) 25 Eucalyptus cordata forest (DCR) 27 Eucalyptus dalrympleana–Eucalyptus pauciflora forest and woodland (DDP) 29 Eucalyptus delegatensis dry forest and woodland (DDE) 31 Eucalyptus globulus dry forest and woodland (DGL) 33 Eucalyptus gunnii woodland (DGW) 35 Eucalyptus morrisbyi forest and woodland (DMO) 37 Eucalyptus nitida dry forest and woodland (DNI) 39 Eucalyptus nitida Furneaux forest (DNF) 41 Eucalyptus obliqua dry forest (DOB) 43 Eucalyptus ovata forest and woodland (DOV) 45 Eucalyptus ovata heathy woodland (DOW) 48 Eucalyptus pauciflora forest and woodland not on dolerite (DPO) 50 Eucalyptus pauciflora forest and woodland on dolerite (DPD) 52 Eucalyptus perriniana forest and woodland (DPE) 54 Eucalyptus pulchella forest and woodland (DPU) 56 Eucalyptus risdonii forest and woodland (DRI) 58 Eucalyptus rodwayi forest and woodland (DRO) 60 Eucalyptus -

Species List
Biodiversity Summary for NRM Regions Species List What is the summary for and where does it come from? This list has been produced by the Department of Sustainability, Environment, Water, Population and Communities (SEWPC) for the Natural Resource Management Spatial Information System. The list was produced using the AustralianAustralian Natural Natural Heritage Heritage Assessment Assessment Tool Tool (ANHAT), which analyses data from a range of plant and animal surveys and collections from across Australia to automatically generate a report for each NRM region. Data sources (Appendix 2) include national and state herbaria, museums, state governments, CSIRO, Birds Australia and a range of surveys conducted by or for DEWHA. For each family of plant and animal covered by ANHAT (Appendix 1), this document gives the number of species in the country and how many of them are found in the region. It also identifies species listed as Vulnerable, Critically Endangered, Endangered or Conservation Dependent under the EPBC Act. A biodiversity summary for this region is also available. For more information please see: www.environment.gov.au/heritage/anhat/index.html Limitations • ANHAT currently contains information on the distribution of over 30,000 Australian taxa. This includes all mammals, birds, reptiles, frogs and fish, 137 families of vascular plants (over 15,000 species) and a range of invertebrate groups. Groups notnot yet yet covered covered in inANHAT ANHAT are notnot included included in in the the list. list. • The data used come from authoritative sources, but they are not perfect. All species names have been confirmed as valid species names, but it is not possible to confirm all species locations. -

Jane Gibbs BA Gdiped Gdippsych
Asthma and Plants: Chemotaxonomic Relationships and Patterns of Asthma Incidence and Respiratory Symptoms, in Urban Coastal Versus Rural Highland Areas in South-East Queensland, Australia, with Special Reference to the Family Myrtaceae Author Gibbs, Jane Published 2007 Thesis Type Thesis (PhD Doctorate) School School of Public Health DOI https://doi.org/10.25904/1912/3128 Copyright Statement The author owns the copyright in this thesis, unless stated otherwise. Downloaded from http://hdl.handle.net/10072/366726 Griffith Research Online https://research-repository.griffith.edu.au ASTHMA AND PLANTS: CHEMOTAXONOMIC RELATIONSHIPS AND PATTERNS OF ASTHMA INCIDENCE AND RESPIRATORY SYMPTOMS, IN URBAN COASTAL VERSUS RURAL HIGHLAND AREAS IN SOUTH-EAST QUEENSLAND, AUSTRALIA, WITH SPECIAL REFERENCE TO THE FAMILY MYRTACEAE. Jane Gibbs BA GDipEd GDipPsych School of Public Health Faculty of Health Science Griffith University Submitted in fulfilment of the requirements of the degree of Doctor of Philosophy on 22 December 2006 ii STATEMENT OF ORIGINALITY This work has not been previously submitted for a degree or diploma in a university. To the best of my knowledge and belief, this thesis contains no material previously published or written by another person except where due reference is made in the thesis itself. Jane Gibbs iii Dedication To my daughter Anna, and all the children who live with asthma, for providing the reason to begin this task, and to her grandmother, and my mother, Marie Gibbs (1926-1995), for providing the persistence required to finish it. iv Abstract This thesis represents an exploratory and iterative study into the relationships of Australian native plants from the family Myrtaceae, with respiratory symptoms, specifically asthma. -

Biodiversity Summary: Burnett Mary, Queensland
Biodiversity Summary for NRM Regions Species List What is the summary for and where does it come from? This list has been produced by the Department of Sustainability, Environment, Water, Population and Communities (SEWPC) for the Natural Resource Management Spatial Information System. The list was produced using the AustralianAustralian Natural Natural Heritage Heritage Assessment Assessment Tool Tool (ANHAT), which analyses data from a range of plant and animal surveys and collections from across Australia to automatically generate a report for each NRM region. Data sources (Appendix 2) include national and state herbaria, museums, state governments, CSIRO, Birds Australia and a range of surveys conducted by or for DEWHA. For each family of plant and animal covered by ANHAT (Appendix 1), this document gives the number of species in the country and how many of them are found in the region. It also identifies species listed as Vulnerable, Critically Endangered, Endangered or Conservation Dependent under the EPBC Act. A biodiversity summary for this region is also available. For more information please see: www.environment.gov.au/heritage/anhat/index.html Limitations • ANHAT currently contains information on the distribution of over 30,000 Australian taxa. This includes all mammals, birds, reptiles, frogs and fish, 137 families of vascular plants (over 15,000 species) and a range of invertebrate groups. Groups notnot yet yet covered covered in inANHAT ANHAT are notnot included included in in the the list. list. • The data used come from authoritative sources, but they are not perfect. All species names have been confirmed as valid species names, but it is not possible to confirm all species locations.