European Parliament 2014-2019
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The New European Parliament: Post-Election Analysis
Heinrich-Böll-Stiftung Europäische Union Rue du Luxembourg,47- 51 - 1050 Brussels, Belgium T+32 2 743 41 00 E [email protected] I eu.boell.org SAVE THE DATE The New European Parliament: Post-Election Analysis From 23 to 26 May, EU citizens will be called to the polls to elect a new European Parliament. The elections will challenge the two largest political groups, as they will most probably face serious losses. The European People's Party (EPP) and the Progressive Alliance of Socialists and Democrats (S&D) are unlikely to hold on to all of their mandates. Far-right and anti-estab- lishment parties could gain more seats than in 2014. Even though the ‘worst case’ scenario of an anti-EU majority is unlikely, the project of European integration might lose momentum as a destructive minority in the European Parliament is likely to grow. On the other hand, smaller groups such as the Liberals and Greens could play a new role in forming majorities in the newly elected Parliament. During the debate, we will examine the election results and analyse voting behaviour in individ- ual EU member states. What will the new European Parliament’s composition look like? What are the implications for coalition-building, the interaction between the EU institutions and the future of the European project? Date Tuesday, 29 May 2019 Time 10 :00 – 12 :00 Venue Atelier29, Rue Jacques de Lalaing 29, 1000 Brussels (Mezzanine) Programme 09:30 – 10:00 Registration & coffee 10:00 – 10:15 Welcome remarks by Eva van de Rakt, Head of Office, Heinrich-Böll-Stiftung European -
Green Deal – the Coordinators
Green Deal – The Coordinators David Sassoli S&D ”I want the European Green Deal to become Europe’s hallmark. At the heart of it is our commitment to becoming the world’s first climate-neutral continent. It is also a long-term economic imperative: those who act first European Parliament and fastest will be the ones who grasp the opportunities from the ecological transition. I want Europe to be 1 February 2020 – H1 2024 the front-runner. I want Europe to be the exporter of knowledge, technologies and best practice.” — Ursula von der Leyen Lorenzo Mannelli Klaus Welle President of the European Commission Head of Cabinet Secretary General Chairs and Vice-Chairs Political Group Coordinators EPP S&D EPP S&D Renew ID Europe ENVI Renew Committee on Europe Dan-Ştefan Motreanu César Luena Peter Liese Jytte Guteland Nils Torvalds Silvia Sardone Vice-Chair Vice-Chair Coordinator Coordinator Coordinator Coordinator the Environment, Public Health Greens/EFA GUE/NGL Greens/EFA ECR GUE/NGL and Food Safety Pacal Canfin Chair Bas Eickhout Anja Hazekamp Bas Eickhout Alexandr Vondra Silvia Modig Vice-Chair Vice-Chair Coordinator Coordinator Coordinator S&D S&D EPP S&D Renew ID Europe EPP ITRE Patrizia Toia Lina Gálvez Muñoz Christian Ehler Dan Nica Martina Dlabajová Paolo Borchia Committee on Vice-Chair Vice-Chair Coordinator Coordinator Coordinator Coordinator Industry, Research Renew ECR Greens/EFA ECR GUE/NGL and Energy Cristian Bușoi Europe Chair Morten Petersen Zdzisław Krasnodębski Ville Niinistö Zdzisław Krasnodębski Marisa Matias Vice-Chair Vice-Chair -

En En Motion for a Resolution
European Parliament 2019-2024 Plenary sitting B9-0271/2021 12.5.2021 MOTION FOR A RESOLUTION to wind up the debate on the statement by the Vice-President of the Commission / High Representative of the Union for Foreign Affairs and Security Policy pursuant to Rule 132(2) of the Rules of Procedure on Chinese countersanctions on EU entities and MEPs and MPs (2021/2644(RSP)) Reinhard Bütikofer, Markéta Gregorová, Viola Von Cramon-Taubadel, Sara Matthieu, Francisco Guerreiro, Alice Kuhnke, Bas Eickhout, Pär Holmgren, Jakop G. Dalunde, Anna Cavazzini, Yannick Jadot, Saskia Bricmont on behalf of the Verts/ALE Group RE\1231430EN.docx PE692.538v01-00 EN United in diversityEN B9-0271/2021 European Parliament resolution on Chinese countersanctions on EU entities and MEPs and MPs (2021/2644(RSP)) The European Parliament, – having regard to its previous resolutions and reports on the situation in China and EU- China relations, in particular those of 21 January 2021 on the crackdown on the democratic opposition in Hong Kong1 and of 17 December 2020 on forced labour and the situation of the Uyghurs in the Xinjiang Uyghur Autonomous Region2 (XUAR), – having regard to its previous recommendations relating to Hong Kong, in particular that of 13 December 2017 to the Council, the Commission and the Vice-President of the Commission / High Representative of the Union for Foreign Affairs and Security Policy (VP/HR) on Hong Kong, 20 years after handover3, – having regard to the statement by leading MEPs following the decision by Chinese authorities to sanction -

Brussels, O3' 03-M Meps Younous Omarjee, Michèle Rivasi, Pascal
Ref. Ares(2017)1147027 - 03/03/2017 European Commission Karmenu ¥ЕЕШ Rue de la Loi, 200 Member of the European Commission B-1049 Brussels Tel. +32 2 295 57 25 [email protected] Brussels, O3' 03-M Ref. Ares(2017)833228 MEPs Younous Omarjee, Michèle Rivasi, Pascal Durand, Bart Staes, Fabio Massimo Castaldo, Bas Eickhout, Estefanía Torres Martínez, Benedek Jávor, Isabella Adinolfi, Davor Skrlec, Georges Bach, Merja Kyllönen, Stefan Eck, Marco Affronte, Nessa Childers, Martin Häusling, Klaus Buchner, Claude Turmes, Ivo Vajgl, Jill Evans, Florent Marcellesi, Sven Giegold, Ernest Urtasun, Emil Radev, Eleonora Evi, Marco Zullo, Guillaume Balas, Claudiu Ciprian Tănăsescu, Jeppe Kofod By email only Dear Members of the European Parliament, Thank you for your letter of 30 January 2017 on Directive 2010/63/EU on the protection of animals used for scientific purposes (here below referred to as "the Directive") and its review obligations. As required by the Directive, the European Commission will be carrying out a review and a feasibility study by 10 November 2017. The review will focus on the early impacts of the Directive based on preliminary findings in selected target areas. This is due to the partly delayed transposition into Member State national legislations and hence still limited experience as regards implementation of the Directive. It is unlikely that the Directive's projected benefits, especially in terms of improved welfare and science, will have fully materialised already this year. However, the Commission has planned another, more comprehensive evaluation for 2019, in line with the Commission Better Regulation programme. In the meantime, extensive stakeholder consultations were held between 27 May and 31 August 2016 inviting the user community (those who breed, supply or use animals), Member State authorities, other EU-level stakeholder organisations and animal welfare organisations at national and at ELI level to participate. -

European Alliance for a Green Recovery
Launch of the European alliance for a Green Recovery Press Release Under embargo until 14/04 7:00am At the initiative of Pascal Canfin, Chair of the Environment Committee at the European Parliament, 180 political decision-makers, business leaders, trade unions, NGOs, and think tanks have come together to form a European alliance for a Green Recovery. In the face of the coronavirus crisis, the biggest challenge Europe has faced in peacetime, with devastating consequences and a shock to the economy tougher than the 2008 crisis, Ministers from 11 countries, 79 cross-party MEPs from 17 Member States, 37 CEOs, 28 business associations representing 10 different sectors, trade union confederation representing members from 90 national trade union organisations and 10 trade union federations, 7 NGOs and 6 think tanks, have committed to working together to create, support and implement solutions to prepare our economies for the world of tomorrow. This first pan-European call for mobilisation on post-crisis green investment packages will work to build the recovery and transformation plans which enshrine the fight against climate change and biodiversity as a key pillar of the economic strategy. Sharing the belief that the economic recovery will only come with massive investments to protect and create jobs and to support all companies, regions and sectors that have suffered from the economy coming to a sudden halt, the alliance commits to contribute to the post-crisis investment decisions needed to reboot and reboost our economy. Covid-19 will not make climate change and nature degradation go away. The fight against this crisis will not be won without a solid economic response. -

Greens/EFA Group - Distribution of Seats in EP Parliamentary Committees
Seats in Committees Update 04.02.2021 Greens/EFA group - Distribution of Seats in EP Parliamentary Committees Parliamentary Committees Seats FULL Members SUBSTITUTE Members Foreign Affairs (AFET) Marketa GREGOROVÁ Alviina ALAMETSÄ Pierrette HERZBERGER- Reinhard BÜTIKOFER FOFANA Viola VON CRAMON Sergey LAGODINSKY 7 Jordi SOLE Katrin LANGENSIEPEN Tineke STRIK Hannah NEUMANN Thomas WAITZ Mounir SATOURI Salima YENBOU Ernest URTASUN Agriculture (AGRI) Claude GRUFFAT Benoit BITEAU 5 Anna DEPARNAY- Francisco GUERREIRO GRUNENBERG Martin HÄUSLING Pär HOLMGREN Bronis ROPĖ Tilly METZ Sarah WIENER Thomas WAITZ Budgets (BUDG) Rasmus ANDRESEN Damien BOESELAGER 4 David CORMAND Henrike HAHN Alexandra GEESE Monika VANA Francisco GUERREIRO Vacant Culture & Education (CULT) Romeo FRANZ Marcel KOLAJA 3 Niklas NIENASS Diana RIBA Salima YENBOU Vacant Development (DEVE) Pierrette HERZBERGER- Alviina ALAMETSÄ FOFANA Benoit BITEAU 3 Erik MARQUARDT Caroline ROOSE Michelle RIVASI Economic & Monetary Affairs Sven GIEGOLD Damien CARÊME (ECON) Claude GRUFFAT Karima DELLI Stasys JAKELIŪNAS Bas EICKHOUT 7 Philippe LAMBERTS Henrike HAHN Kira PETER-HANSEN Ville NIINISTÖ Ernest URTASUN Mikulas PEKSA Piernicola PEDICINI Vacant Committee seats - UPDATE 30.9.20 Employment & Social Affairs Kira PETER-HANSEN Romeo FRANZ 4 (EMPL) Katrin LANGENSIEPEN Terry REINTKE Mounir SATOURI Kim VAN SPARRENTAK Tatjana ŽDANOKA Sara MATTHIEU Environment, Public Health & Margarete AUKEN Michael BLOSS Food safety (ENVI) Bas EICKHOUT Manuela RIPA Pär HOLMGREN Sven GIEGOLD Yannick JADOT Martin HÄUSLING -

12.3.2019 B8-0171/2019 } B8-0172/2019 } B8-0173/2019 } B8-0174/2019 } RC1/Am
12.3.2019 B8-0171/2019 } B8-0172/2019 } B8-0173/2019 } B8-0174/2019 } RC1/Am. 2 Amendment 2 Philippe Lamberts, Terry Reintke, Ernest Urtasun, Molly Scott Cato, Bas Eickhout, Thomas Waitz, Sven Giegold on behalf of the Verts/ALE Group Joint motion for a resolution PPE, S&D, ECR, ALDE, Verts/ALE Gender balance in EU economic and monetary affairs nominations Joint motion for a resolution Citation 19 Joint motion for a resolution Amendment – having regard to Rule 123(2) and (4) of – having regard to Rule 123(2) and its Rules of Procedure, (4) and Rule 228a of its Rules of Procedure, Or. en AM\1179489EN.docx PE635.430v01-00 } PE635.431v01-00 } PE635.432v01-00 } PE635.433v01-00 } RC1 EN United in diversityEN 12.3.2019 B8-0171/2019 } B8-0172/2019 } B8-0173/2019 } B8-0174/2019 } RC1/Am. 3 Amendment 3 Philippe Lamberts, Terry Reintke, Ernest Urtasun, Molly Scott Cato, Bas Eickhout, Thomas Waitz, Sven Giegold on behalf of the Verts/ALE Group Joint motion for a resolution PPE, S&D, ECR, ALDE, Verts/ALE Gender balance in EU economic and monetary affairs nominations Joint motion for a resolution Paragraph 1 a (new) - before 1 Joint motion for a resolution Amendment 1a. Deeply regrets the lack of female candidates in the shortlists put forward for the positions of Chair of the European Banking Authority (EBA), and member of the Single Resolution Board (SRB) and of the Executive Board of the European Central Bank (ECB); calls, therefore, for a postponement of the votes on Parliament’s decisions on the proposals of the Commission for the appointment of a member of the SRB and of the Executive Board of the ECB and for the rejection of the appointment of the Chairperson of the EBA; Or. -

Brussels, 17 April 2020 To: Emily O'reilly European Ombudsperson
Brussels, 17 April 2020 To: Emily O’Reilly European Ombudsperson Object: Contract awarded by the European Commission to BlackRock Dear Ms O’Reilly, We would like to raise our concerns regarding the contract won by BlackRock for a study to the European Commission on how the EU could best integrate environmental, social and governance (ESG) factors into its banking supervision, as reported for example by the Guardian on 12th of April 20201. This contract - with a total value of EUR 280,000 – raises, in our views, issues of conflicts of interests. Following the complaint submitted by Damien Carême, Member of the European Parliament on behalf of the signatories below, we would like you to investigate several aspects of this tendering procedure. First of all, we would like to ensure that all the rules have been properly followed by the Commission services in the attribution of this public tender2 to BlackRock. This could be checked through an inspection from your office if necessary. Secondly, we would like to ensure that the Directive 2014/24/EU on public procurement3 - in particular its Articles 24 and 57 - have been properly respected by the Commission. We would like to ensure that the Commission has properly looked into the conflict of interest raised by BlackRock. Taken together, BlackRock funds are indeed among the world’s largest investors in banks and fossil fuel companies. More precisely, this asset management company is a top-three investor in all eight of the world’s largest oil companies, and a top-10 investor in the 12 most systemically important banks in the world. -

Brussels, 24 February 2021
Brussels, 24 February 2021 Declaration from Members of the European Parliament to urge the Commission and Member States not to block the TRIPS waiver at the WTO and to support global access to COVID-19 vaccines We, Members of the European Parliament, urge the European Commission and the European Council to review their opposition to the TRIPS waiver proposal at the World Trade Organisation (WTO), which serves to enable greater access to affordable COVID-19 health technologies, including vaccines, in particular for developing and middle income countries. This call comes in view of the European Council meeting of 25 February 2021 and the crucial decision to be made by all Member States at the WTO General Council on 1-2 March 2021. Since the beginning of the pandemic, the need to ensure global open access to COVID-19 health technologies and to rapidly scale up their manufacturing and supply has been widely acknowledged. However, despite efforts and statements made by the European Commission and several heads of state in support of treating COVID-19 medical products as global public goods, this has not yet translated into actionable realities. In this context, the EU’s open opposition to the TRIPS waiver risks exacerbating a dangerous North-South divide when it comes to affordable access to COVID-19 diagnostics, personal protective equipment, treatments and vaccines. The WTO decision on a potential waiver offers a crucial and much-needed act of effective solidarity, as it is an important step towards increasing local production in partner countries and, ultimately, suppressing this pandemic on a global scale. -

1 03 Sept. 2014 How the Sakharov Prize 2014 Is Awarded Background Briefing in the Next 10 Days, the Nominations of the Sakharov
1 03 Sept. 2014 How the Sakharov Prize 2014 is awarded Background briefing In the next 10 days, the nominations of the Sakharov Prize for Human Rights will be decided by the European Parliament. The prize is awarded to “honour exceptional individuals who combat intolerance, fanaticism and oppression.”1 Previous winners include Nelson Mandela, Reporters without Borders and Anatoli Marchenko. If you believe that Azerbaijani human rights defenders – who are now in jail following years of work on behalf of the rights of others, and most recently on a list of political prisoners in Azerbaijan (on which they are now included) – then let the MEPs who vote on this know. Nominations for the Sakharov Prize can be made by: Political groups in the European Parliament. Or At least 40 MEPs. The deadline for nominations is Thursday 18 September at 12:00 in Strasbourg. NOTE: In order to decide on a nominee from their group some political groups have internal deadlines in the course of the next week. The next days are crucial. We focus here on four important political groups which might to support this nomination: The EPP Social Democrats Liberals Greens 1Source: The European Union website: http://www.europarl.europa.eu/aboutparliament/en/00f3dd2249/Sakharov-Prize-for-Freedom-of-Thought.html 2 Once the nominations are been made, the Foreign Affairs and Development committees vote on a shortlist of three finalists. This happens on either Monday 6th or Tuesday 7th October 2014. The members list for the foreign affairs committee can be found here: http://www.europarl.europa.eu/committees/en/afet/members.html, while the development committee is here: http://www.europarl.europa.eu/committees/en/deve/members.html#menuzone. -

1 Presiding Officer of the Scottish Parliament Ken
Presiding Officer of the Scottish Parliament Ken Macintosh MSP Scottish Parliament Edinburgh EH99 1SP 8 April 2017 Subject: 50 POST 50 Dear Presiding Officer, Dear Members of the Scottish Parliament, As elected representatives from across the European Union we have been heartened by your support for a Europe which is united in pursuit of progress and not divided by fear and self-interest. Democracy and mutual respect are at the heart of the European project and so, whilst we are saddened by the vote of a small majority for the United Kingdom to leave the EU, we respect this as a democratic decision of UK citizens. We recognise that this was not your choice however and that Scotland voted strongly to remain within the EU. The question of Scotland’s constitutional future, and your relationships with the UK and the EU are for the people of Scotland to decide. It is not our place to tell Scotland what path you should take. We regret that the UK's government has chosen to follow the path of a 'hard Brexit' and has so far refused to properly take into account the preferences of Scottish citizens in the withdrawal process. Therefore, if Scotland were to become an independent country and decided to seek to maintain European Union membership, we offer our full support to ensure the transition is as swift, smooth, and orderly as possible. Scotland would be most welcome as a full member of the European Union, with your five million European citizens continuing to benefit from the rights and protections we all currently enjoy. -
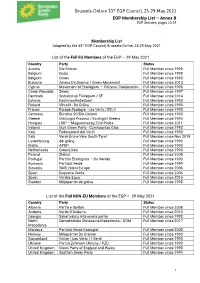
EGP Membership List, Annex B of the EGP Statutes
Brussels-Online 33rd EGP Council, 25-29 May 2021 EGP Membership List – Annex B EGP Statutes, pages 23-25 Membership List Adopted by the 33rd EGP Council, Brussels-Online, 25-29 May 2021 List of the Full EU Members of the EGP – 29 May 2021 Country Party Status Austria Die Grünen Full Member since 1993 Belgium Ecolo Full Member since 1993 Belgium Groen Full Member since 1993 Bulgaria Zeleno Dvizheniye / Green Movement Full Member since 2013 Cyprus Movement of Ecologists — Citizens' Cooperation Full Member since 1998 Czech Republic Zelení Full Member since 1997 Denmark Socialistisk Folkeparti / SF Full Member since 2014 Estonia Eestimaa Rohelised Full Member since 1993 Finland Vihreät - De Gröna Full Member since 1993 France Europe Ecologie - Les Verts / EELV Full Member since 1993 Germany Bündnis 90/Die Grünen Full Member since 1993 Greece Oikologoi-Prasinoi / Ecologist Greens Full Member since 1994 Hungary LMP – Magyarország Zöld Pártja Full Member since 2011 Ireland Irish Green Party - Comhaontas Glas Full Member since 1993 Italy Federazione dei Verdi Full Member since 1993 Italy Verdi-Grüne-Vërc South Tyrol Full Member since Nov 2019 Luxembourg déi gréng Full Member since 1993 Malta APDP Full Member since 1993 Netherlands GroenLinks Full Member since 1993 Poland Zieloni Full Member since 2005 Portugal Partido Ecologista – Os Verdes Full Member since 1993 Romania Partidul Verde Full Member since 1999 Slovenia SMS Zeleni Evrope Full Member since 2006 Spain Esquerra Verda Full Member since 2006 Spain Verdes Equo Full Member since 2016 Sweden