Blattodea Karyotype Database
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

New Aspects About Supella Longipalpa (Blattaria: Blattellidae)
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector Asian Pac J Trop Biomed 2016; 6(12): 1065–1075 1065 HOSTED BY Contents lists available at ScienceDirect Asian Pacific Journal of Tropical Biomedicine journal homepage: www.elsevier.com/locate/apjtb Review article http://dx.doi.org/10.1016/j.apjtb.2016.08.017 New aspects about Supella longipalpa (Blattaria: Blattellidae) Hassan Nasirian* Department of Medical Entomology and Vector Control, School of Public Health, Tehran University of Medical Sciences, Tehran, Iran ARTICLE INFO ABSTRACT Article history: The brown-banded cockroach, Supella longipalpa (Blattaria: Blattellidae) (S. longipalpa), Received 16 Jun 2015 recently has infested the buildings and hospitals in wide areas of Iran, and this review was Received in revised form 3 Jul 2015, prepared to identify current knowledge and knowledge gaps about the brown-banded 2nd revised form 7 Jun, 3rd revised cockroach. Scientific reports and peer-reviewed papers concerning S. longipalpa and form 18 Jul 2016 relevant topics were collected and synthesized with the objective of learning more about Accepted 10 Aug 2016 health-related impacts and possible management of S. longipalpa in Iran. Like the Available online 15 Oct 2016 German cockroach, the brown-banded cockroach is a known vector for food-borne dis- eases and drug resistant bacteria, contaminated by infectious disease agents, involved in human intestinal parasites and is the intermediate host of Trichospirura leptostoma and Keywords: Moniliformis moniliformis. Because its habitat is widespread, distributed throughout Brown-banded cockroach different areas of homes and buildings, it is difficult to control. -

UNIVERSITY of CALIFORNIA RIVERSIDE Isolation
UNIVERSITY OF CALIFORNIA RIVERSIDE Isolation, Determination of Absolute Stereochemistry, and Asymmetric Synthesis of Insect Methyl-Branched Hydrocarbons A Dissertation submitted in the partial satisfaction of the requirements for the degree of Doctor of Philosophy in Chemistry by Jan Edgar Bello June 2014 Dissertation Committee: Dr. Jocelyn G. Millar, Chairperson Dr. Thomas H. Morton Dr. Catharine Larsen Copyright by Jan Edgar Bello 2014 The Dissertation of Jan Edgar Bello is approved: ________________________________________________________________ ________________________________________________________________ ________________________________________________________________ Committee Chairperson University of California, Riverside Acknowledgements This dissertation would not have been possible without the guidance, assistance, and support from both my academic and biological families. I would first and foremost like to thank my advisor Professor Jocelyn G. Millar, who has guided me through this rigorous process and has helped me become the chemical ecologist I am today. I would also like to thank my research group Dr. Steve McElfresh, Dr. Yunfan Zou, Dr. Rebeccah Waterworth, R. Max Collignon, Joshua Rodstein, Jackie Serrano, and Brian Hanley for all the suggestions, insect collecting, synthetic discussions, and experimental advise that have allowed me to complete this dissertation. I would like to send a huge thank you to my family who have always believed in me. To my mom and dad, thank you for your encouragement, for loving me, and for your support (both financial and emotional). To my siblings, Jonathan, Michelle, and John-C thank you for your encouragement and for praying for me, especially during the beginning of my PhD studies when things were overwhelming. I would also like to thank my friends, Ryan Neff, Lauren George, Jenifer N. -

A New Insect Trackway from the Upper Jurassic—Lower Cretaceous Eolian Sandstones of São Paulo State, Brazil: Implications for Reconstructing Desert Paleoecology
A new insect trackway from the Upper Jurassic—Lower Cretaceous eolian sandstones of São Paulo State, Brazil: implications for reconstructing desert paleoecology Bernardo de C.P. e M. Peixoto1,2, M. Gabriela Mángano3, Nicholas J. Minter4, Luciana Bueno dos Reis Fernandes1 and Marcelo Adorna Fernandes1,2 1 Laboratório de Paleoicnologia e Paleoecologia, Departamento de Ecologia e Biologia Evolutiva, Universidade Federal de São Carlos (UFSCar), São Carlos, São Paulo, Brazil 2 Programa de Pós Graduacão¸ em Ecologia e Recursos Naturais, Centro de Ciências Biológicas e da Saúde, Universidade Federal de São Carlos (UFSCar), São Carlos, São Paulo, Brazil 3 Department of Geological Sciences, University of Saskatchewan, Saskatoon, Saskatchewan, Canada 4 School of the Environment, Geography, and Geosciences, University of Portsmouth, Portsmouth, Hampshire, United Kingdom ABSTRACT The new ichnospecies Paleohelcura araraquarensis isp. nov. is described from the Upper Jurassic-Lower Cretaceous Botucatu Formation of Brazil. This formation records a gigantic eolian sand sea (erg), formed under an arid climate in the south-central part of Gondwana. This trackway is composed of two track rows, whose internal width is less than one-quarter of the external width, with alternating to staggered series, consisting of three elliptical tracks that can vary from slightly elongated to tapered or circular. The trackways were found in yellowish/reddish sandstone in a quarry in the Araraquara municipality, São Paulo State. Comparisons with neoichnological studies and morphological inferences indicate that the producer of Paleohelcura araraquarensis isp. nov. was most likely a pterygote insect, and so could have fulfilled one of the Submitted 6 November 2019 ecological roles that different species of this group are capable of performing in dune Accepted 10 March 2020 deserts. -

Cómo Citar El Artículo Número Completo Más Información Del
Acta zoológica mexicana ISSN: 0065-1737 ISSN: 2448-8445 Instituto de Ecología A.C. García, Mauricio; Camacho, Jesús; Dorado, Idelma Dos nuevas especies de Termitaradus Myers, 1924 (Hemiptera: Termitaphididae), de Venezuela y observaciones sobre la familia Acta zoológica mexicana, vol. 32, núm. 3, 2016, pp. 348-358 Instituto de Ecología A.C. Disponible en: http://www.redalyc.org/articulo.oa?id=57549165012 Cómo citar el artículo Número completo Sistema de Información Científica Redalyc Más información del artículo Red de Revistas Científicas de América Latina y el Caribe, España y Portugal Página de la revista en redalyc.org Proyecto académico sin fines de lucro, desarrollado bajo la iniciativa de acceso abierto ISSN 0065-1737 (NUEVA SERIE) 32(3) 2016 DOS NUEVAS ESPECIES DE TERMITARADUS MYERS, 1924 (HEMIPTERA: TERMITAPHIDIDAE), DE VENEZUELA Y OBSERVACIONES SOBRE LA FAMILIA TWO NEW SPECIES OF TERMITARADUS MYERS, 1924 (HEMIPTERA: TERMITAPHIDIDAE) OF VENEZUELA, AND OBSERVATIONS ON THE FAMILY Mauricio GARCÍA,¹ Jesús CAMACHO² e Idelma DORADO² ¹ Centro de Investigaciones Biológicas (CIB), Facultad de Humanidades y Educación, Edificio de Postgrado, Universidad del Zulia, Apdo. 526, A-4001, Venezuela. ² Museo de Artrópodos de la Universidad del Zulia (MALUZ). Departamento Fitosanitario, Facultad de Agronomía, Universidad del Zulia, Apdo. 526, Maracaibo A-4001, Zulia, Venezuela. <[email protected]>; <[email protected]>; <[email protected]> Recibido: 28/03/2016; aceptado: 30/08/2016 Editor asociado responsable: Alfonso Neri García Aldrete García, M., Camacho, J. & Dorado, I. (2016). Dos nuevas especies García, M., Camacho, J. & Dorado, I. (2016). Two new species of de Termitaradus Myers, 1924 (Hemiptera: Termitaphididae), de Termitaradus Myers, 1924 (Hemiptera: Termitaphididae) of Ven- Venezuela y observaciones sobre la familia. -

Cockroach Marion Copeland
Cockroach Marion Copeland Animal series Cockroach Animal Series editor: Jonathan Burt Already published Crow Boria Sax Tortoise Peter Young Ant Charlotte Sleigh Forthcoming Wolf Falcon Garry Marvin Helen Macdonald Bear Parrot Robert E. Bieder Paul Carter Horse Whale Sarah Wintle Joseph Roman Spider Rat Leslie Dick Jonathan Burt Dog Hare Susan McHugh Simon Carnell Snake Bee Drake Stutesman Claire Preston Oyster Rebecca Stott Cockroach Marion Copeland reaktion books Published by reaktion books ltd 79 Farringdon Road London ec1m 3ju, uk www.reaktionbooks.co.uk First published 2003 Copyright © Marion Copeland All rights reserved No part of this publication may be reproduced, stored in a retrieval system or transmitted, in any form or by any means, electronic, mechanical, photocopying, recording or otherwise without the prior permission of the publishers. Printed and bound in Hong Kong British Library Cataloguing in Publication Data Copeland, Marion Cockroach. – (Animal) 1. Cockroaches 2. Animals and civilization I. Title 595.7’28 isbn 1 86189 192 x Contents Introduction 7 1 A Living Fossil 15 2 What’s in a Name? 44 3 Fellow Traveller 60 4 In the Mind of Man: Myth, Folklore and the Arts 79 5 Tales from the Underside 107 6 Robo-roach 130 7 The Golden Cockroach 148 Timeline 170 Appendix: ‘La Cucaracha’ 172 References 174 Bibliography 186 Associations 189 Websites 190 Acknowledgements 191 Photo Acknowledgements 193 Index 196 Two types of cockroach, from the first major work of American natural history, published in 1747. Introduction The cockroach could not have scuttled along, almost unchanged, for over three hundred million years – some two hundred and ninety-nine million before man evolved – unless it was doing something right. -

The Termite by Ogden Nash
Biological studies on two European termite species: establishment risk in the UK Laetitia Virginie Laine B.Sc. M.Sc. D.I.C. A thesis submitted for the degree of Doctor of Philosophy of the University of London November 2002 Department of Biological Sciences, Imperial College, Silwood Park, Ascot, SL5 7PY, Berkshire Abstract The discovery of an accidental introduction of termites into Devon in 1994 generated great interest as termites were previously thought to be unable to establish in the UK due to unfavourable climatic conditions. Information about the species present in Devon, Reticulitermes grassei, was found to be lacking and the present study was undertaken to determine the importance of various abiotic and biotic factors in establishment of this species. The factors included in the study were the minimum termite number for establishment, the consumption of wood and its effect on survival and temperature and soil type. A review of the literature was also conducted, detailing the problems with the taxonomy of this termite genus, their present distribution pattern and the life cycle of Reticulitermes species. Two populations of both R. grassei and R. santonensis were studied. The effect of the minimum termite number was found to be significant in both laboratory and field conditions. However, survival decreased in the laboratory and increased in the field with increased number of termites. Consumption experiments were performed using blocks of Scots pine, beech and oak. In most cases termite populations were found to consume and survive best on oak. Consumption was also tested on live seedlings but these results were inconclusive. Survival was observed to increase with increased temperature. -
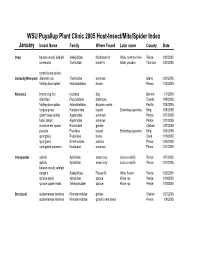
Host Insect List 2005
WSU Puyallup Plant Clinic 2005 Host-Insect/Mite/Spider Index January Insect Name Family Where Found Latin name County Date Crop balsam woolly adelgid Adelgididae Nordmann fir Abies nordmannian Pierce 1/31/2005 coneworm Tortricidae noble fir Abies procera Thurston 1/31/2005 comb footed spider, Curiosity/Non-pest Steatoda sp. Theridiidae unknown Island 1/21/2005 folding-door spider Antrodiaetidae house Pierce 1/12/2005 Nuisance brown dog tick Ixodidae dog Benton 1/7/2005 drainflies Psychodidae bathroom Cowlitz 1/28/2005 folding-door spider Antrodiaetidae daycare center Pacific 1/24/2005 fungus gnats Fungivoridae loquat Eriobotrya japonica King 1/24/2005 giant house spider Agelenidae unknown Pierce 1/21/2005 hobo spider Agelenidae unknown Pierce 1/21/2005 moisture ant queen Formicidae garden Clallam 1/27/2005 psocids Psocidae loquat Eriobotrya japonica King 1/24/2005 springtails Poduridae home Clark 1/10/2005 springtails Sminthuridae outside Pierce 1/26/2005 variegated cutworm Noctuidae unknown Pierce 1/21/2005 Ornamental aphids Aphididae sweet bay Laurus nobilis Pierce 1/27/2005 aphids Aphididae sweet bay Laurus nobilis Pierce 1/27/2005 balsam woolly adelgid nymphs Adelgididae Frasier fir Abies fraseri Pierce 1/25/2005 spruce aphid Aphididae spruce Picea sp. Pierce 1/10/2005 spruce spider mites Tetranchyidae spruce Picea sp. Pierce 1/10/2005 Structural subterranean termites Rhinotermitidae garden Clallam 1/27/2005 subterranean termites Rhinotermitidae ground near shed Pierce 1/8/2005 February Insect Name Family Where Found Latin name County -

Isoptera Book Chapter
Isoptera 535 See Also the Following Articles Biodiversity ■ Biogeographical Patterns ■ Cave Insects ■ Introduced Insects Further Reading Carlquist , S. ( 1974 ) . “ Island Biology . ” Columbia University Press , New York and London . Gillespie , R. G. , and Roderick , G. K. ( 2002 ) . Arthropods on islands: Colonization, speciation, and conservation . Annu. Rev. Entomol. 47 , 595 – 632 . Gillespie , R. G. , and Clague , D. A. (eds.) (2009 ) . “ Encyclopedia of Islands. ” University of California Press , Berkeley, CA . Howarth , F. G. , and Mull , W. P. ( 1992 ) . “ Hawaiian Insects and Their Kin . ” University of Hawaii Press , Honolulu, HI . MacArthur , R. H. , and Wilson , E. O. ( 1967 ) . “ The Theory of Island Biogeography . ” Princeton University Press , Princeton, NJ . Wagner , W. L. , and Funk , V. (eds.) ( 1995 ) . “ Hawaiian Biogeography Evolution on a Hot Spot Archipelago. ” Smithsonian Institution Press , Washington, DC . Whittaker , R. J. , and Fern á ndez-Palacios , J. M. ( 2007 ) . “ Island Biogeography: Ecology, Evolution, and Conservation , ” 2nd ed. Oxford University Press , Oxford, U.K . I Isoptera (Termites) Vernard R. Lewis FIGURE 1 Castes for Isoptera. A lower termite group, University of California, Berkeley Reticulitermes, is represented. A large queen is depicted in the center. A king is to the left of the queen. A worker and soldier are he ordinal name Isoptera is of Greek origin and refers to below. (Adapted, with permission from Aventis Environmental the two pairs of straight and very similar wings that termites Science, from The Mallis Handbook of Pest Control, 1997.) Thave as reproductive adults. Termites are small and white to tan or sometimes black. They are sometimes called “ white ants ” and can be confused with true ants (Hymenoptera). -

Dictyoptera: Blattaria: Polyphagidae) from Korea Reveal About Cryptocercus Evolution? a Study in Morphology, Molecular Phylogeny, and Chemistry of Tergal Glands
PROCEEDINGS OF THE ACADEMY OF NATURAL SCIENCES OF PHILADELPHIA 151: 61±79. 31 DECEMBER 2001 What does Cryptocercus kyebangensis, n.sp. (Dictyoptera: Blattaria: Polyphagidae) from Korea reveal about Cryptocercus evolution? A study in morphology, molecular phylogeny, and chemistry of tergal glands PHILIPPE GRANDCOLAS,1 YUNG CHUL PARK,2 JAE C. CHOE,3 MARIA-DOLORS PIULACHS,3 XAVIER BELLEÂS,3 CYRILLE D'HAESE,1 JEAN-PIERRE FARINE,4 AND REÂMY BROSSUT4 1ESA 8043 CNRS, Laboratoire d'Entomologie, MuseÂum national d'Histoire naturelle, 45, rue Buffon, 75005 Paris, FranceÐ [email protected] 2School of Biological Sciences, Seoul National University, Kwanak-ku Shilim-dong San 56-1, Seoul 151-742, South Korea 3Department of Physiology and Molecular Biodiversity, Institut de Biologia Molecular de Barcelona (CSIC), Jordi Girona 18, 0834 Barcelona, Spain 4UMR 5548 CNRS, Faculte des Sciences, Universite de Bourgogne, 6, bd. Gabriel, 21000 Dijon, France ABSTRACTÐThe description of a new species of the woodroach Cryptocercus kyebangensis Grandcolas from South Korea offers the opportunity to bring comparative information within the genus. This species, though morphologically very similar to other East Asian and North American species, presents conspicuous differentiation of both ribosomal genes (sequenced fragments of 12S and 16S) and chemical blends from tergal glands (proportions of linalyl acetate and the alcohol 4, 6, 8-trimethyl-7, 9- undecadien-5-ol, compounds previously identi®ed in females originating from North America). A phylogenetic reconstruction involving Blatta orientalis as an outgroup, Therea petiveriana as a polyphagid relative, C. kyebangensis and 17 North American Cryptocercus populations showed that C. kyebangensis stands as a sister-group of North American Cryptocercus, thus suggesting that one beringian vicariance has taken place in the early differentiation of the genus. -

Evaluation of the Chemical Defense Fluids of Macrotermes Carbonarius
www.nature.com/scientificreports OPEN Evaluation of the chemical defense fuids of Macrotermes carbonarius and Globitermes sulphureus as possible household repellents and insecticides S. Appalasamy1,2*, M. H. Alia Diyana2, N. Arumugam2 & J. G. Boon3 The use of chemical insecticides has had many adverse efects. This study reports a novel perspective on the application of insect-based compounds to repel and eradicate other insects in a controlled environment. In this work, defense fuid was shown to be a repellent and insecticide against termites and cockroaches and was analyzed using gas chromatography-mass spectrometry (GC– MS). Globitermes sulphureus extract at 20 mg/ml showed the highest repellency for seven days against Macrotermes gilvus and for thirty days against Periplaneta americana. In terms of toxicity, G. sulphureus extract had a low LC50 compared to M. carbonarius extract against M. gilvus. Gas chromatography–mass spectrometry analysis of the M. carbonarius extract indicated the presence of six insecticidal and two repellent compounds in the extract, whereas the G. sulphureus extract contained fve insecticidal and three repellent compounds. The most obvious fnding was that G. sulphureus defense fuid had higher potential as a natural repellent and termiticide than the M. carbonarius extract. Both defense fuids can play a role as alternatives in the search for new, sustainable, natural repellents and termiticides. Our results demonstrate the potential use of termite defense fuid for pest management, providing repellent and insecticidal activities comparable to those of other green repellent and termiticidal commercial products. A termite infestation could be silent, but termites are known as destructive urban pests that cause structural damage by infesting wooden and timber structures, leading to economic loss. -

Treatise on the Isoptera of the World Kumar
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by American Museum of Natural History Scientific Publications KRISHNA ET AL.: ISOPTERA OF THE WORLD: 7. REFERENCES AND INDEX7. TREATISE ON THE ISOPTERA OF THE WORLD 7. REFERENCES AND INDEX KUMAR KRISHNA, DAVID A. GRIMALDI, VALERIE KRISHNA, AND MICHAEL S. ENGEL A MNH BULLETIN (7) 377 2 013 BULLETIN OF THE AMERICAN MUSEUM OF NATURAL HISTORY TREATISE ON THE ISOPTERA OF THE WORLD VolUME 7 REFERENCES AND INDEX KUMAR KRISHNA, DAVID A. GRIMALDI, VALERIE KRISHNA Division of Invertebrate Zoology, American Museum of Natural History Central Park West at 79th Street, New York, New York 10024-5192 AND MICHAEL S. ENGEL Division of Invertebrate Zoology, American Museum of Natural History Central Park West at 79th Street, New York, New York 10024-5192; Division of Entomology (Paleoentomology), Natural History Museum and Department of Ecology and Evolutionary Biology 1501 Crestline Drive, Suite 140 University of Kansas, Lawrence, Kansas 66045 BULLETIN OF THE AMERICAN MUSEUM OF NATURAL HISTORY Number 377, 2704 pp., 70 figures, 14 tables Issued April 25, 2013 Copyright © American Museum of Natural History 2013 ISSN 0003-0090 2013 Krishna ET AL.: ISOPtera 2435 CS ONTENT VOLUME 1 Abstract...................................................................... 5 Introduction.................................................................. 7 Acknowledgments . 9 A Brief History of Termite Systematics ........................................... 11 Morphology . 44 Key to the -

Diversity and Abundance of Subterranean Termites in South India
Srinivasa Murthy, K. Available Ind. J. Pure online App. Biosci.at www.ijpab.com (2020) 8(5), 141 -149 ISSN: 2582 – 2845 DOI: http://dx.doi.org/10.18782/2582-2845.8193 ISSN: 2582 – 2845 Ind. J. Pure App. Biosci. (2020) 8(5), 141-149 Research Article Peer-Reviewed, Refereed, Open Access Journal Diversity and abundance of Subterranean Termites in South India K. Srinivasa Murthy* National Bureau of Agricultural Insect Resources, P B No. 2491, H A Farm Post, Bellary Road Bangalore - 560 024, Karnataka, India *Corresponding Author E-mail: [email protected] Received: 7.07.2020 | Revised: 12.08.2020 | Accepted: 20.08.2020 ABSTRACT The abundance and diversity of subterranean termites was studied in the states of Andhra Pradesh, Keralae, Karnataka and Tamilnadu. Fifteen species of termites belonging to subfamilies Apicotermitinae, Kalotermitidae, Macrotermitinae and Nasutitermitinae, were recorded. The fungus growing termites (Macrotermitinae) accounted for 66.66% abundance, across the states. The Apicotermitinae (soil feeders) and Kalotermitidae (dry wood termites) registered 6.62% each and the dry wood termites (Nasutitermitinae) recorded 20.1% abundance. Among the different species of termites, Odontermes obesus, was more predominant (15.62%) than others. The cropping pattern, soil type and topography predisposed the abundance and diversity of termites. Keywords: Abundance, Cropping pattern, Diversity, Macrotermitinae. INTRODUCTION Ali, et al., 2013) as they play a vital role in Termites (Isoptera) are considered as the most recycling of plant materials and wood, abundant invertebrates and represent up to modifying and improving the soil condition 95% of soil insect biomass show an elaborated and composition, and providing food for other morphology and complex behaviour (Wang, et animals (Ackerman et al.