Commercial Biotechnology: an International Analysis (January 1984)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Biosensors and Bioelectronics 142 (2019) 111530
Biosensors and Bioelectronics 142 (2019) 111530 Contents lists available at ScienceDirect Biosensors and Bioelectronics journal homepage: www.elsevier.com/locate/bios Highly sensitive bioaffinity electrochemiluminescence sensors: Recent T advances and future directions ∗ Bahareh Babamiria,b, Delnia Baharia,b, Abdollah Salimia,b,c, a Department of Chemistry, University of Kurdistan, 66177-15175, Sanandaj, Iran b Research Center for Nanotechnology, University of Kurdistan, 66177-15175, Sanandaj, Iran c Department of Chemistry, University of Western Ontario, N6A 5B7, London, Ontario, Canada ARTICLE INFO ABSTRACT Keywords: Electrogenerated chemiluminescence (also called electrochemiluminescence and abbreviated ECL) has attracted Electrochemiluminescence much attention in various fields of analysis due to the potential remarkably high sensitivity, extremely wide Bioaffinity sensors dynamic range and excellent controllability. Electrochemiluminescence biosensor, by taking the advantage of Aptasensor the selectivity of the biological recognition elements and the high sensitivity of ECL technique was applied as a Immunoassays powerful analytical device for ultrasensitive detection of biomolecule. In this review, we summarize the latest Genosensor sensing applications of ECL bioanalysis in the field of bio affinity ECL sensors including aptasensors, im- Cytosensor Medical diagnostics munoassays and DNA analysis, cytosensor, molecularly imprinted sensors, ECL resonance energy transfer and Nanomaterials ratiometric biosensors and give future perspectives for new developments in ECL analytical technology. Furthermore, the results herein discussed would demonstrate that the use of nanomaterials with unique chemical and physical properties in the ECL biosensing systems is one of the most interesting research lines for the development of ultrasensitive electrochemiluminescence biosensors. In addition, ECL based sensing assays for clinical samples analysis and medical diagnostics and developing of immunosensors, aptasensors and cytosensor for this purpose is also highlighted. -
Accomplishments in Nanotechnology
U.S. Department of Commerce Carlos M. Gutierrez, Secretaiy Technology Administration Robert Cresanti, Under Secretaiy of Commerce for Technology National Institute ofStandards and Technolog}' William Jeffrey, Director Certain commercial entities, equipment, or materials may be identified in this document in order to describe an experimental procedure or concept adequately. Such identification does not imply recommendation or endorsement by the National Institute of Standards and Technology, nor does it imply that the materials or equipment used are necessarily the best available for the purpose. National Institute of Standards and Technology Special Publication 1052 Natl. Inst. Stand. Technol. Spec. Publ. 1052, 186 pages (August 2006) CODEN: NSPUE2 NIST Special Publication 1052 Accomplishments in Nanoteciinology Compiled and Edited by: Michael T. Postek, Assistant to the Director for Nanotechnology, Manufacturing Engineering Laboratory Joseph Kopanski, Program Office and David Wollman, Electronics and Electrical Engineering Laboratory U. S. Department of Commerce Technology Administration National Institute of Standards and Technology Gaithersburg, MD 20899 August 2006 National Institute of Standards and Teclinology • Technology Administration • U.S. Department of Commerce Acknowledgments Thanks go to the NIST technical staff for providing the information outlined on this report. Each of the investigators is identified with their contribution. Contact information can be obtained by going to: http ://www. nist.gov Acknowledged as well, -

Bioelectronics Contents
chapter 10 Bioelectronics Contents Introduction . 253 Biosensors. 253 Biochips. 254 Priorities for Future Research . 256 Chapter IO References . 256 Figure Figure No. Page 29.The Use of Proteins in Constructing Circuit . ..., . 255 — Chapter 10 Bioelectronics .—— .— Introduction --—----- ——— The potential for the use of proteins in elec- used for several years, but design problems have tronic dmices has received attention recently with limited their acceptance. Biotechnology is ex- the advent of recombinant DNA (rDNA) technol- pected to increase the variety, stability, and sen- ogy}’ and the potential for computer-aided design sitivity of these devices. Biochips (biologically of proteins (I ,2,3 5 ,6,7, 11, 13,14,15,19,2 1 ). Work based microchips) capable of logic and memory is focused in two areas: biosensors and biochips. are still only speculative, and their development Biosensors (biological}’ based sensors) have been is many years away. Biosensors -——-- –—- —— .——. A potential application of biotechnology is in the not only could obviate the need for enzymes but development of improved sensing devices. Be- also could substantially broaden the applications cause of their high specificity. for given sub- of biosensors. A longer term solution to the lack of stances, enzymes and monocIonal antibodies particular enzymes might be to have computers (hlAbs) are particularly suited for use as sensors. design enzymes with particular catalytic func- Sensors using these biological molecules have the tions. Finally, features of proteins that determine potential to be smaller and more sensitive than temperature stability could be incorporated into traditional sensors. the genes that code for important sensing en- zymes. Biosensors using enzymes have been used to detect the presence of various organic compounds A new approach to fabrication is yielding bio- for many years (12). -

Universidade Federal Do Rio Grande Do Sul Faculdade
UNIVERSIDADE FEDERAL DO RIO GRANDE DO SUL FACULDADE DE AGRONOMIA PROGRAMA DE PÓS-GRADUAÇÃO EM ZOOTECNIA FATORES INTRÍNSECOS À PRODUÇÃO, O USO DA INSEMINAÇÃO ARTIFICIAL E OS OBJETIVOS DE SELEÇÃO NA PECUÁRIA LEITEIRA DO SUL DO BRASIL Heitor José Cervo Médico Veterinário/UFSM Mestre em Clínica de Grandes Animais/UFSM Tese apresentada como um dos requisitos à obtenção do grau de Doutor em Zootecnia Área de concentração Produção Animal Porto Alegre (RS), Brasil Outubro, 2014 CIP – Catalogação na Publicação C419f Cervo, Heitor José Fatores intrínsecos à produção, o uso da inseminação artificial e os objetivos de seleção na pecuária leiteira do sul do Brasil / Heitor José Cervo. – 2014. 214 f. ; 30 cm. Tese (Doutorado em Zootecnia) – Universidade Federal do Rio Grande do Sul, Faculdade de Agronomia, Porto Alegre, RS, 2014. Orientadora: Concepta Margaret McManus Pimentel. Coorientador: Júlio Otávio Jardim Barcellos. 1. Bovino de leite - Produção. 2. Bovino de leite - Inseminação artificial. 3. Produção animal. I. Pimentel, Concepta Margaret McManus, orientadora. II. Barcellos, Júlio Otávio Jardim, coorientador. III. Título. CDU: 636.2 Catalogação: Bibliotecária Jucelei Rodrigues Domingues - CRB 10/1569 2 3 AGRADECIMENTOS Agradeço a Deus pelo credo de nossa alma! A minha família, em especial a minha esposa Gilda Maria Saldanha Fernandez e meus filhos, Carlos Heitor Fernandez Cervo e Vitória Fernandez Cervo, pelo apoio de forma incondicional e cumplicidade para a busca de qualquer objetivo. A Universidade Federal do Rio Grande do Sul, pela oportunidade de cursar doutorado em uma instituição de excelência e propiciar o crescimento pessoal. Ao Instituto Federal do Rio Grande do Sul, pelo estímulo para a busca de novos conhecimentos necessários a esta instituição e a sociedade que servimos. -
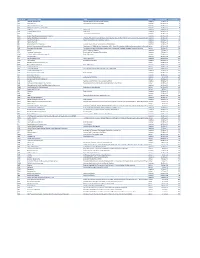
Mvx List.Pdf
MVX_CODE manufacturer_name Notes status last updated date manufacturer_id AB Abbott Laboratories includes Ross Products Division, Solvay Inactive 16-Nov-17 1 ACA Acambis, Inc acquired by sanofi in sept 2008 Inactive 28-May-10 2 AD Adams Laboratories, Inc. Inactive 16-Nov-17 3 ALP Alpha Therapeutic Corporation Inactive 16-Nov-17 4 AR Armour part of CSL Inactive 28-May-10 5 AVB Aventis Behring L.L.C. part of CSL Inactive 28-May-10 6 AVI Aviron acquired by Medimmune Inactive 28-May-10 7 BA Baxter Healthcare Corporation-inactive Inactive 28-May-10 8 BAH Baxter Healthcare Corporation includes Hyland Immuno, Immuno International AG,and North American Vaccine, Inc./acquired somInactive 16-Nov-17 9 BAY Bayer Corporation Bayer Biologicals now owned by Talecris Inactive 28-May-10 10 BP Berna Products Inactive 28-May-10 11 BPC Berna Products Corporation includes Swiss Serum and Vaccine Institute Berne Inactive 16-Nov-17 12 BTP Biotest Pharmaceuticals Corporation New owner of NABI HB as of December 2007, Does NOT replace NABI Biopharmaceuticals in this codActive 28-May-10 13 MIP Emergent BioSolutions Formerly Emergent BioDefense Operations Lansing and Michigan Biologic Products Institute Active 16-Nov-17 14 CSL bioCSL bioCSL a part of Seqirus Inactive 26-Sep-16 15 CNJ Cangene Corporation Purchased by Emergent Biosolutions Inactive 29-Apr-14 16 CMP Celltech Medeva Pharmaceuticals Part of Novartis Inactive 28-May-10 17 CEN Centeon L.L.C. Inactive 28-May-10 18 CHI Chiron Corporation Part of Novartis Inactive 28-May-10 19 CON Connaught acquired by Merieux Inactive 28-May-10 21 DVC DynPort Vaccine Company, LLC Active 28-May-10 22 EVN Evans Medical Limited Part of Novartis Inactive 28-May-10 23 GEO GeoVax Labs, Inc. -

An Aptamer-Based Magnetic Flow Cytometer Using Matched Filtering
Biosensors and Bioelectronics 169 (2020) 112362 Contents lists available at ScienceDirect Biosensors and Bioelectronics journal homepage: http://www.elsevier.com/locate/bios An aptamer-based magnetic flow cytometer using matched filtering Chih-Cheng Huang a, Partha Ray b, Matthew Chan c, Xiahan Zhou c, Drew A. Hall c,d,* a Materials Science and Engineering Program, University of California – San Diego, La Jolla, CA, 92093, USA b Division of Surgical Oncology, Department of Surgery, Moores Cancer Center, UC San Diego Health, La Jolla, CA, 92093, USA c Department of Electrical and Computer Engineering, University of California – San Diego, La Jolla, CA, 92093, USA d Department of Bioengineering, University of California – San Diego, La Jolla, CA, 92093, USA ARTICLE INFO ABSTRACT Keywords: Facing unprecedented population-ageing, the management of noncommunicable diseases (NCDs) urgently needs Aptasensor a point-of-care (PoC) testing infrastructure. Magnetic flow cytometers are one such solution for rapid cancer Flow cytometry cellular detection in a PoC setting. In this work, we report a giant magnetoresistive spin-valve (GMR SV) Magnetic biosensor biosensor array with a multi-stripe sensor geometry and matched filteringto improve detection accuracy without Matched filtering compromising throughput. The carefully designed sensor geometry generates a characteristic signature when Pancreatic cancer Point-of-Care (PoC) testing cells labeled with magnetic nanoparticles (MNPs) pass by thus enabling multi-parametric measurement like optical flow cytometers (FCMs). Enumeration and multi-parametric information were successfully measured across two decades of throughput (37 — 2730 cells/min). 10-μm polymer microspheres were used as a bio mimetic model where MNPs and MNP-decorated polymer conjugates were flown over the GMR SV sensor array and detected with a signal-to-noise ratio (SNR) as low as 2.5 dB due to the processing gain afforded by the matched filtering. -

Biotechnology Past, Present and Potential
4i-"l 11- HONORARY FELLOW'S ADDRESS TO IFST 20TH ANNIVERSARY SYMPOSIUM - 16 OCT. 1985 MANCHESTER, ENGLAND IDRC - 1-lb BIOTECHNOLOGY PAST, PRESENT AND POTENTIAL BY: JOSEPH H. HULSE* BIOTECHNOLOGY: ANCIENT AND MODERN Louis Pasteur wrote "There are no applied sciences; there are only applications of science...The study of the application of science is very easy to anyone who is master of the theory". A few years later Lord Kelvin instructed us that "If you can measure that of which you speak, and can express it by a number, you know something of your subject. But if you cannot measure it, your knowledge is meagre and unsatisfactory." It would indeed be interesting to know what the spirits of these distinguished scientists are thinkinq about "Biotechnology" which takes within its broad embrace remarkable new knowledge in cell and molecular biology; some very ancient technologies; together with a large swatch of enpirical observations and discoveries, many of which remain far distant from viable technological application. Fermentation technologies have a very long history: beer, wine, bread and cheese having been around as long as cereals and vine fruits have been harvested and animais have been milked. Homer described wine as a qift from the Gods and Ecclesiasticus wisely advised that "From the beginning wine was created to make men joyful, not to make them drunk." Though ethanolic fermentations have been most pervasive, lactic and other acidic fermentations have appeared in greater diversity, particularly in traditional domestic processes of preservation. The ancient Sumerians 7,000 years ago converted all their milk into cheese in the stated belief that had God intended mankind to have clean milk to drink he would have placed the udders at the front end of the cow. -

Bioelectronics & Nanotechnology
Bioelectronics & Nanotechnology A Master Degree in Biomedical Sciences – two years – full-time/part-time This unique master degree programme focuses on a novel and interdisciplinary scientific domain at the boundaries between physics, chemistry, electro- nics, and biomedical sciences. Fifty years ago, shortly after J. Watson and F. Crick successfully solved the structure of DNA using X-ray diffraction, R. Feynman made his visionary statement: “There’s plenty of room at the bottom”, with which he effectively heralded the new era of nanosciences. Since that time, research into “functi- onal” molecules and nanomaterials has developed into one of the most important scientific disciplines of today. This is triggered by the fact that a broad range of nanoscopic techniques has become available to study molecules directly at their own length scale and to understand their complex properties. The master programme offers a strong foundation in all fundamental scientific aspects and provides, in addition, an in depth introduction into several important application areas. Topics range from integrated detection and characterization techniques for molecules (biosensors) to the nanoscale engi- neering of implant materials, and the working principles of medical devices like neurochips and pacemakers. The curriculum of this programme was jointly developed by physicists, chemists, clinical and biomedical researchers, and engineers specialized in medi- cal instrumentation. The programme ensures therefore a broad overview of the domain of bioelectronics and nanotechnology and enables the graduates to develop their individual skills for a successful career in interdisciplinary research- and development environments. What are your career prospects ? Educational Concept • Applied and fundamental research at universities, hospitals, and research In view of the international profile of this degree programme, English langu- centers. -

Science & Technology Trends 2020-2040
Science & Technology Trends 2020-2040 Exploring the S&T Edge NATO Science & Technology Organization DISCLAIMER The research and analysis underlying this report and its conclusions were conducted by the NATO S&T Organization (STO) drawing upon the support of the Alliance’s defence S&T community, NATO Allied Command Transformation (ACT) and the NATO Communications and Information Agency (NCIA). This report does not represent the official opinion or position of NATO or individual governments, but provides considered advice to NATO and Nations’ leadership on significant S&T issues. D.F. Reding J. Eaton NATO Science & Technology Organization Office of the Chief Scientist NATO Headquarters B-1110 Brussels Belgium http:\www.sto.nato.int Distributed free of charge for informational purposes; hard copies may be obtained on request, subject to availability from the NATO Office of the Chief Scientist. The sale and reproduction of this report for commercial purposes is prohibited. Extracts may be used for bona fide educational and informational purposes subject to attribution to the NATO S&T Organization. Unless otherwise credited all non-original graphics are used under Creative Commons licensing (for original sources see https://commons.wikimedia.org and https://www.pxfuel.com/). All icon-based graphics are derived from Microsoft® Office and are used royalty-free. Copyright © NATO Science & Technology Organization, 2020 First published, March 2020 Foreword As the world Science & Tech- changes, so does nology Trends: our Alliance. 2020-2040 pro- NATO adapts. vides an assess- We continue to ment of the im- work together as pact of S&T ad- a community of vances over the like-minded na- next 20 years tions, seeking to on the Alliance. -

Presentation Title Slide, No Image: One-Line Title Preferred
Analysis of the Global TB Drug Market and Country-Specific Case Studies of TB Drug Distribution Channels UK Case Study Prepared with IMS Consulting November 2006 Country table of contents • TB Control in the UK • Procurement and Distribution of TB Drugs • Value and Volume of the UK TB Market •Appendix 2 TB Control in the UK After years of increases, the incidence of TB in the UK has only recently begun to plateau • Prevalence and incidence of TB has been rising in the UK for more than 15 years • This has been attributed to increased migration from countries with high TB burden • The ageing UK population and increase in HIV/AIDS has also contributed Prevalence and incidence of TB and HIV/AIDS in the UK Distribution of age groups in the UK in 1971 RATE OF ANNUAL PERCENTAGE and 2004 NUMBER NUMBER OF TB CHANGE IN TB YEAR OF TB HIV/AIDS (PER NO. OF Age 1971 2004 CASES RATE CASES 100 000) CASES group 1999 5761 10.8 - - 41 585 Under 16 25% 19% 2000 6323 11.8 +9.8 +9.4 45 449 16-65 62% 65% 2001 6652 12.4 +5.2 +4.8 50 511 Over 65 13% 16% 2002 6861 12.7 +3.1 +2.4 56 738 2003 6837 12.5 -0.3 -1.0 64 005 Source: Health Protection Agency; www.avert.org; Office of National Statistics 3 T B C o TB casesnt are concentrated in in ro has by far thel in highest number of cases th e U K Prevalence and incid 3500 3000 S ASE2500 C F 2000 O R E B 1500 t M h U e U N 1000 K 500 (2003)e nce of TB across 0 London ner city areas -- West Midlands North West Sour East Midlands c e : Health Protecti Yorkshire&Humber 45 • South East T 40 he largest proportion (45%) of TB cas East of England 35 o n R Agen located in London 30 A • South West T E 25 ( c A London y P e North East 20 E majority of scases repo in the UK R occur in inner cities (there is 15 100 almost no incidence in rural Wales 10 000) locations). -

Consolidation Revisited Is the Founder of Managing Resources, a Date Received *In Revised Form) 8Th October, 2001 Consultancy Focused on the Life Science Industries
Alan Williams Consolidation revisited is the founder of Managing Resources, a Date received *in revised form) 8th October, 2001 consultancy focused on the life science industries. The Alan Williams company undertakes strategic assignments and also assists public and private sector clients with regulatory affairs, investor Abstract In 1998, the author produced two papers that argued that consolidation was relations and human a necessary activity for biotechnology companies to pay greater attention to. Three resources development. years later a further consideration of the subject seems worthwhile. Examples of Alan has also been an investment manager in successful consolidators are reviewed. a venture capital company, and held Keywords: consolidation, mergers, acquisitions marketing and licensing positions in Fisons. Introduction drink, vaccines and animal health but there is now greater focus on the highest In the early/mid-1970s, the pharmaceutical pro®tability sector, human pharmaceuticals. industry consisted of more than 100 At the time of writing, GSK is rumoured to research-based businesses of some be a possible purchaser of Bayer's signi®cant size, although none of them pharmaceutical business, the future of could convincingly claim a global presence. which has been questioned after the Typically, a leading company had a strong withdrawal of cerivastatin. position in its local market "examples Aventis, the eighth largest pharmaceutical included Merck and P®zer in the USA, company by sales in 2000,1 includes the Bayer in Germany, Beecham in the UK, operations of 1970s companies such as Roussel-Uclaf in France and Fujisawa in Rhone-Poulenc, Rorer, Fisons, Hoechst, Japan) and possibly some other Marion, Richardson Merrell, and some neighbouring countries "US companies in smaller companies. -

LISTA LUCRARI Cercetător Ştiinţific III Dr. Andra OROS A.TEZA DE DOCTORAT Titlu: “CONTRIBUTII LA CUNOASTEREA CONSECINTELOR
Concurs pentru ocuparea postului de cercetator ştiinţific II Disciplina: Oceanografie Chimica LISTA LUCRARI Cercetãtor ştiinţific III dr. Andra OROS A.TEZA DE DOCTORAT Titlu: “CONTRIBUTII LA CUNOASTEREA CONSECINTELOR POLUÃRII CU METALE GRELE ASUPRA ECOSISTEMELOR MARINE COSTIERE DE LA LITORALUL ROMANESC AL MÃRII NEGRE”. (197 pag.) Specializarea “Ecologie si protecţia mediului” în cadrul Facultãţii de Ştiinţele Naturii, Universitatea “Ovidius” Constanţa. Perioada : 2001-2009. Conducãtor ştiinţific : Prof. Dr. Marian – Traian Gomoiu, Membru Corespondent al Academiei Române. -Rezumat Teza de doctorat (36p), publicat online: http://ro.scribd.com/doc/92593292/Rezumat-Oros (865 vizualizari). B. CARTI -STATE OF THE ENVIRONMENT OF THE BLACK SEA (2001-2006/7) / Capitolul 3 “The state of the chemical pollution”. Korshenko A, Denga Y, Gvakharia B, Machitadze N, Oros A. Edited by Temel Oguz. Publications on the Protection of the Black Sea Against Pollution (BSC) 2008-3, Istanbul, Turkey, 448 pp, 2008. ISBN 978-9944-245-33-3. (disponibila si online: http://www.blacksea-commission.org/_publ-SOE2009.asp) -IDENTIFIED GAPS ON MSFD ASSESSMENT ELEMENTS. PERSEUS PROJECT, 2013, 72p. Laroche Sophie, Andral Bruno, Cadiou Jean-François, Pantazi Maria, Gonzalez Fernandez Daniel, Vasile Daniela, Vasilopoulou Vassiliki Celia, Hanke Georg, Secrieru Dan, Marian Traian Gomoiu, Oaie Gheorghe, Begun Tatiana, Galgani François, Rougeron Natacha, Lorance Pascal, Tsangaris Catherine, Aristides Prospathopoulos, Symboura Nomiki, Kontogiannis Charilaos, Tsagarakis Konstantinos, Boicenco Laura, Dumitrache Camelia, Lazar Luminita, Oros Andra, Coatu Valentina, Radu Gheorghe, Moncheva Snejana. Funding from the European Community’s Seventh Framework Programme (FP7/2007- 2013) under grant agreement no 287600 – project PERSEUS (Policy – oriented marine Environmental Research for the Southern European Seas). ISBN 978-960-9783-01-3 (disponibila si online: http://www.perseus-net.eu/site/content.php?locale=1&locale_j=en&sel=558).