Caenorhabditis Elegans
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

| Sydney Brenner |
| SYDNEY BRENNER | TOP THREE AWARDS • Nobel Prize in Physiology, 2002 • Albert Lasker Special Achievement Award, 2000 • National Order of Mapungubwe (Gold), 2004 DEFINING MOMENT To view the DNA model for the first time. 32 |LEGENDS OF SOUTH AFRICAN SCIENCE| A LIFE DEDICATED TO SCIENCE C. ELEGANS WORK In the more than eight decades that Nobel Laureate, Prof Sydney Brenner, “To start with we propose to identify every cell in the worm and trace line- has all-consumingly devoted his life to science, he twice wrote powerful age. We shall also investigate the constancy of development and study proposals of no longer than a page. Short but sweet, these kick-started the its control by looking for mutants,” is how Brenner ended his proposal on two projects that are part of his lasting legacy. Caenorhabditis elegans to the UK Medical Research Council in October 1963. He was looking for a new challenge after already having helped to The first was to request funding to study a worm, because he saw in the show that genetic code is composed of non-overlapping triplets and that nematode Caenorhabditis elegans the ideal genetic model organism. messenger ribonucleic acid (mRNA) exists. He was right, and received the Nobel Prize for his efforts. The other pro- posal, which set out how Singapore could become a hub for biomedical His first paper on C. elegans appeared in Genetics in 1974, and in all, the research, earned him the title of “mentor to a nation’s science ambitions”. work took about 20 years to reach its full potential. -

Cambridge's 92 Nobel Prize Winners Part 4 - 1996 to 2015: from Stem Cell Breakthrough to IVF
Cambridge's 92 Nobel Prize winners part 4 - 1996 to 2015: from stem cell breakthrough to IVF By Cambridge News | Posted: February 01, 2016 Some of Cambridge's most recent Nobel winners Over the last four weeks the News has been rounding up all of Cambridge's 92 Nobel Laureates, which this week comes right up to the present day. From the early giants of physics like JJ Thomson and Ernest Rutherford to the modern-day biochemists unlocking the secrets of our genome, we've covered the length and breadth of scientific discovery, as well as hugely influential figures in economics, literature and politics. What has stood out is the importance of collaboration; while outstanding individuals have always shone, Cambridge has consistently achieved where experts have come together to bounce their ideas off each other. Key figures like Max Perutz, Alan Hodgkin and Fred Sanger have not only won their own Nobels, but are regularly cited by future winners as their inspiration, as their students went on to push at the boundaries they established. In the final part of our feature we cover the last 20 years, when Cambridge has won an average of a Nobel Prize a year, and shows no sign of slowing down, with ground-breaking research still taking place in our midst today. The Gender Pay Gap Sale! Shop Online to get 13.9% off From 8 - 11 March, get 13.9% off 1,000s of items, it highlights the pay gap between men & women in the UK. Shop the Gender Pay Gap Sale – now. Promoted by Oxfam 1.1996 James Mirrlees, Trinity College: Prize in Economics, for studying behaviour in the absence of complete information As a schoolboy in Galloway, Scotland, Mirrlees was in line for a Cambridge scholarship, but was forced to change his plans when on the weekend of his interview he was rushed to hospital with peritonitis. -

Lasker Interactive Research Nom'18.Indd
THE 2018 LASKER MEDICAL RESEARCH AWARDS Nomination Packet albert and mary lasker foundation November 1, 2017 Greetings: On behalf of the Albert and Mary Lasker Foundation, I invite you to submit a nomination for the 2018 Lasker Medical Research Awards. Since 1945, the Lasker Awards have recognized the contributions of scientists, physicians, and public citizens who have made major advances in the understanding, diagnosis, treatment, cure, and prevention of disease. The Medical Research Awards will be offered in three categories in 2018: Basic Research, Clinical Research, and Special Achievement. The Lasker Foundation seeks nominations of outstanding scientists; nominations of women and minorities are encouraged. Nominations that have been made in previous years are not automatically reconsidered. Please see the Nomination Requirements section of this booklet for instructions on updating and resubmitting a nomination. The Foundation accepts electronic submissions. For information on submitting an electronic nomination, please visit www.laskerfoundation.org. Lasker Awards often presage future recognition of the Nobel committee, and they have become known popularly as “America’s Nobels.” Eighty-seven Lasker laureates have received the Nobel Prize, including 40 in the last three decades. Additional information on the Awards Program and on Lasker laureates can be found on our website, www.laskerfoundation.org. A distinguished panel of jurors will select the scientists to be honored with Lasker Medical Research Awards. The 2018 Awards will -

Advertising (PDF)
Share the wonders of the brain and mind with A PUBLIC INFORMATION INITIATIVE OF: Seeking resources to communicate with the public about neuroscience? Educating others through Brain Awareness activities? BrainFacts.org can help you communicate how the brain works. Explore BrainFacts.org for easy-to-use, accessible resources including: s Information about hundreds of diseases and disorders s Concepts about brain function s Educational tools s Multimedia tools and a social media community s Interviews and discussions with leading researchers; and more Visit BrainFacts.org Give to the Friends of SfN Fund Join us in forging the future of neuroscience Support a future of discovery and progress through travel awards and public education and outreach programs. To inquire about specific initiatives or to make a tax-deductible contribution, visit SfN.org or email: [email protected]. THE HISTORY OF NEUROSCIENCE IN AUTOBIOGRAPHY THE LIVES AND DISCOVERIES OF EMINENT SENIOR NEUROSCIENTISTS CAPTURED IN AUTOBIOGRAPHICAL BOOKS AND VIDEOS The History of Neuroscience in Autobiography Series Edited by Larry R. Squire Outstanding neuroscientists tell the stories of their scientific work in this fascinating series of autobiographical essays. Within their writings, they discuss major events that shaped their discoveries and their influences, as well as people who inspired them and helped shape their careers as neuroscientists. The History of Neuroscience in Autobiography, Vol. 1 The History of Neuroscience in Autobiography, Vol. 4 Denise Albe-Fessard, Julius Axelrod, Peter O. Bishop, Per Andersen, Mary Bunge, Jan Bures, Jean-Pierre Changeux, Theodore H. Bullock, Irving T. Diamond, Robert Galambos, John Dowling, Oleh Hornykiewicz, Andrew Huxley, Jac Sue Viktor Hamburger, Sir Alan L. -

Discovery of the Secrets of Life Timeline
Discovery of the Secrets of Life Timeline: A Chronological Selection of Discoveries, Publications and Historical Notes Pertaining to the Development of Molecular Biology. Copyright 2010 Jeremy M. Norman. Date Discovery or Publication References Crystals of plant and animal products do not typically occur naturally. F. Lesk, Protein L. Hünefeld accidentally observes the first protein crystals— those of Structure, 36;Tanford 1840 hemoglobin—in a sample of dried menstrual blood pressed between glass & Reynolds, Nature’s plates. Hunefeld, Der Chemismus in der thierischen Organisation, Robots, 22.; Judson, Leipzig: Brockhaus, 1840, 158-63. 489 In his dissertation Louis Pasteur begins a series of “investigations into the relation between optical activity, crystalline structure, and chemical composition in organic compounds, particularly tartaric and paratartaric acids. This work focused attention on the relationship between optical activity and life, and provided much inspiration and several of the most 1847 HFN 1652; Lesk 36 important techniques for an entirely new approach to the study of chemical structure and composition. In essence, Pasteur opened the way to a consideration of the disposition of atoms in space.” (DSB) Pasteur, Thèses de Physique et de Chimie, Presentées à la Faculté des Sciences de Paris. Paris: Bachelier, 1847. Otto Funcke (1828-1879) publishes illustrations of crystalline 1853 hemoglobin of horse, humans and other species in his Atlas der G-M 684 physiologischen Chemie, Leizpig: W. Englemann, 1853. Charles Darwin and Alfred Russel Wallace publish the first exposition of the theory of natural selection. Darwin and Wallace, “On the Tendency of 1858 Species to Form Varieties, and on the Perpetuation of Varieties and G-M 219 Species by Natural Means of Selection,” J. -

The Sui Generis Sydney Brenner,” by Thoru Pederson, Which Was First Published June 10, 2019; 10.1073/ Pnas.1907536116 (Proc
Correction RETROSPECTIVE Correction for “The sui generis Sydney Brenner,” by Thoru Pederson, which was first published June 10, 2019; 10.1073/ pnas.1907536116 (Proc. Natl. Acad. Sci. U.S.A. 116,13155–13157). The author notes that, on page 13155, left column, second paragraph, lines 13–14, “Cyril Hinshelwood at Oxford, a leading figure in the early bacteriophage field” has been revised to read “Cyril Hinshelwood at Oxford, a chemist turned bacteriologist.” Additionally, in the original version of this article, the author stated that the experiments performed by Brenner and Crick involved chemically induced mutations in given DNA letters. In fact, most of the data were from spontaneous mutations. We have removed this incorrect assertion. The online version of the article has been corrected. Published under the PNAS license. Published online July 15, 2019. www.pnas.org/cgi/doi/10.1073/pnas.1910910116 CORRECTION www.pnas.org PNAS | July 23, 2019 | vol. 116 | no. 30 | 15307 Downloaded by guest on October 1, 2021 RETROSPECTIVE The sui generis Sydney Brenner RETROSPECTIVE Thoru Pedersona,1 Sydney Brenner died on April 5, 2019, at age 92. His fame arose from three domains in which he operated with uncommon intellectual vibrancy. First were his prescient ideas and breakthrough experiments that defined the DNA genetic code and how the informa- tion it contains is transmitted into proteins. Second, in a later career, he developed a model organism, the roundworm Caenorhabditis elegans, to determine how the cells of an animal descend, one by one, along pathways of increasing specialization. Last was his be- guiling skill as an intellectual sharpshooter, often sur- prising colleagues by the immediacy of his “take” of a problem, even ones somewhat beyond his ken. -
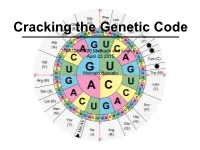
MCDB 5220 Methods and Logics April 23 2015 Marcelo Bassalo
Cracking the Genetic Code MCDB 5220 Methods and Logics April 23 2015 Marcelo Bassalo Last Tuesday… Nirenberg and Matthaei: RNA is the template for protein synthesis (poly-U —> phenylalanine) Thursday! Francis Crick Sydney Brenner • 1927 - today • Born in South Africa • BS in Anatomy and Physiology • MS in Cytogenetics • PhD in Physical Chemistry from Oxford • Joined Salk Institute in 1976 • Established C. elegans as model organism for developmental biology • 2002 Nobel Prize Physiology or Medicine Leslie Barnett • 1920 - 2002 • Born in London • BS in Dairying • Worked with Brenner for most part of her life Cracking the Genetic Code Bacteriophage T4 Escherichia coli Figure from Brock Biology of Microorganisms Cracking the Genetic Code Viral Plaques Why Phage T4? • Plaques are an easy screening system • Allows investigation of rare events (trillions of tries in a single LB plate) • rII locus: phenotypes allows genetic mapping (Benzer) rII genes E. coli K-12 (K) E. coli B (B) WT ✓ ✓ A B non-leaky larger/irregular plaques mutation (r-plaque) (null) X A B leaky larger/irregular plaques mutation ✓ (r-plaque) (partial function) A B Why Phage T4? B’ B’/B’’ Grow in B null mutation No growth in K Grow in B null mutation Growth in K B’’ WT { Grow in B No growth in K # plaques (K) Distance (B’ to B’’) = # plaques (B) The Genetic Code is not overlapping Evidence comes from previous studies: • Tobacco mosaic virus RNA: mutations in RNA change only 1 amino acid (Tsugita et. al) • Abnormal human hemoglobins shows only single amino acid changes (Watson -
Nobel Laureates in Physiology Or Medicine
All Nobel Laureates in Physiology or Medicine 1901 Emil A. von Behring Germany ”for his work on serum therapy, especially its application against diphtheria, by which he has opened a new road in the domain of medical science and thereby placed in the hands of the physician a victorious weapon against illness and deaths” 1902 Sir Ronald Ross Great Britain ”for his work on malaria, by which he has shown how it enters the organism and thereby has laid the foundation for successful research on this disease and methods of combating it” 1903 Niels R. Finsen Denmark ”in recognition of his contribution to the treatment of diseases, especially lupus vulgaris, with concentrated light radiation, whereby he has opened a new avenue for medical science” 1904 Ivan P. Pavlov Russia ”in recognition of his work on the physiology of digestion, through which knowledge on vital aspects of the subject has been transformed and enlarged” 1905 Robert Koch Germany ”for his investigations and discoveries in relation to tuberculosis” 1906 Camillo Golgi Italy "in recognition of their work on the structure of the nervous system" Santiago Ramon y Cajal Spain 1907 Charles L. A. Laveran France "in recognition of his work on the role played by protozoa in causing diseases" 1908 Paul Ehrlich Germany "in recognition of their work on immunity" Elie Metchniko France 1909 Emil Theodor Kocher Switzerland "for his work on the physiology, pathology and surgery of the thyroid gland" 1910 Albrecht Kossel Germany "in recognition of the contributions to our knowledge of cell chemistry made through his work on proteins, including the nucleic substances" 1911 Allvar Gullstrand Sweden "for his work on the dioptrics of the eye" 1912 Alexis Carrel France "in recognition of his work on vascular suture and the transplantation of blood vessels and organs" 1913 Charles R. -

The Promise of Synthetic Biology
Building Living Machines with Biobricks The Promise of Synthetic Biology Professor Richard Kitney, Professor Paul Freemont Support by Matthieu Bultelle, Dr Robert Dickinson, Dr Suhail Islam, Kirsten Jensen, Paul Kitney, Dr Duo Lu, Dr Chueh Loo Poh, Vincent Rouilly, Baojun Wang. The Fathers of Biological Engineering Imperial College London Department of Bioengineering Division of Molecular Biosciences Norbert Wiener American Mathematician (1894–1964) Throughout his life Wiener had many extra- mathematical interests, especially in biology and philosophy. At Harvard his studies in philosophy led him to an interest in mathematical logic and this was the subject of his doctoral thesis, which he completed at the age of 18. In 1920 he joined the faculty of the Massachusetts Institute of Technology, where he became professor of mathematics (1932). He made significant contributions to a number of areas of mathematics including harmonic analysis and Fourier transforms. Norbert Wiener is best known for his theory of cybernetics, the comparative study of control and communication in humans and machines. He also made significant contributions to the development of computers and calculators. The Feedback Loop: A crucial concept of Cybernetics Claude Elwood Shannon American Mathematician (1916–2001) Shannon graduated from the University of Michigan in 1936. He later worked both at the Massachusetts Institute of Technology and the Bell Telephone Laboratories. In 1958 he returned to MIT as Donner Professor of Science, held until his retirement in 1978. Shannon's greatest contribution was in laying the mathematical foundations of communication theory. The resulting theory found applications in such wide-ranging fields as circuit design, communication technology in general, and even in biology, psychology, semantics, and linguistics. -

SYDNEY BRENNER Salk Institute, 100010 N
NATURE’S GIFT TO SCIENCE Nobel Lecture, December 8, 2002 by SYDNEY BRENNER Salk Institute, 100010 N. Torrey Pines Road, La Jolla, California, USA, and King’s College, Cambridge, England. The title of my lecture is “Nature’s gift to Science.” It is not a lecture about one scientific journal paying respects to another, but about how the great di- versity of the living world can both inspire and serve innovation in biological research. Current ideas of the uses of Model Organisms spring from the ex- emplars of the past and choosing the right organism for one’s research is as important as finding the right problems to work on. In all of my research these two decisions have been closely intertwined. Without doubt the fourth winner of the Nobel prize this year is Caenohabditis elegans; it deserves all of the honour but, of course, it will not be able to share the monetary award. I intend to tell you a little about the early work on the nematode to put it into an intellectual perspective. It bridges, both in time and concept, the biol- ogy we practice today and the biology that was initiated some fifty years ago with the revolutionary discovery of the double-helical structure of DNA by Watson and Crick. My colleagues who follow will tell you more about the worm and also recount their incisive research on the cell lineage and on the genetic control of all death. To begin with, I can do no better than to quote from the paper I published in 1974 (1). -

TRINITY 2005 Contents
Fusion #4f 10/6/05 1:42 pm Page 2 fusionTHE NEWSLETTER OF THE SIR WILLIAM DUNN SCHOOL OF PATHOLOGY ISSUE 4 . TRINITY 2005 Contents Editorial 1 Editorial News 2 Anyone reading William James’s article on the history of Virology at the Dunn School Virology at the will not fail to notice his ‘aside’ speculation on why the Dunn School has been such a Dunn School 4 successful contributor to medical science and fundamental biology. It has always been an Institute without Divisions, and has consequently fostered strong interdisciplinary Albert Beyers 6 science. You, Me and HIV 7 Our strong publication record and success in attract senior figures in the areas of the raising grant funding attests to the success of molecular basis of cancer, and in cell-signalling. Jim Gowans that philosophy. This is, of course, a structure To achieve the former the Division has agreed to recirculated 8 which carries some risks, as certain important allow the department to fundraise for the Research Notes 9 areas may go through periods when the "active creation of the César Milstein Chair in Molecular Basis of Cancer. César Milstein was an old friend mass" in a given area would seem small. In Overcoming antibiotic recent years the department has had the of the department, and his discovery of resistance 10 satisfaction of seeing that some of its star monoclonal antibodies opened up many fields of scientists have moved to occupy important medical science for which many of his friends Fishing for leadership positions in other British Universities here are enormously grateful. -

Single-Molecule FRET Reveals a Corkscrew RNA Structure for the Polymerase-Bound Influenza Virus Promoter
Single-molecule FRET reveals a corkscrew RNA PNAS PLUS structure for the polymerase-bound influenza virus promoter Alexandra I. Tomescua,1, Nicole C. Robba,1, Narin Hengrungb,c, Ervin Fodorb,2, and Achillefs N. Kapanidisa,2 aBiological Physics Research Group, Clarendon Laboratory, Department of Physics, University of Oxford, Oxford OX1 3PU, United Kingdom; bSir William Dunn School of Pathology, University of Oxford, Oxford OX1 3RE, United Kingdom; and cDivision of Structural Biology, Wellcome Trust Centre for Human Genetics, University of Oxford, Oxford OX3 7BN, United Kingdom Edited by Robert A. Lamb, Northwestern University, Evanston, IL, and approved July 8, 2014 (received for review April 1, 2014) The influenza virus is a major human and animal pathogen re- development of specific antiviral agents, such as decoy RNAs sponsible for seasonal epidemics and occasional pandemics. The (10). Despite this, little structural information is available re- genome of the influenza A virus comprises eight segments of single- garding the mechanisms of RNA promoter recognition and stranded, negative-sense RNA with highly conserved 5′ and 3′ ter- binding by the influenza virus RNAP. mini. These termini interact to form a double-stranded promoter During the influenza virus life cycle, the viral RNAP tran- structure that is recognized and bound by the viral RNA-dependent scribes the vRNA genome into capped and polyadenylated RNA polymerase (RNAP); however, no 3D structural information for mRNAs using short primers containing a 5′ 7-methyl-guanosine the influenza polymerase-bound promoter exists. Functional studies cap structure derived from host cell pre-mRNAs. The vRNA have led to the proposal of several 2D models for the secondary segments are also replicated by the RNAP via cRNA inter- structure of the bound promoter, including a corkscrew model in mediates, which in turn are used as templates to make more ′ ′ which the 5 and 3 termini form short hairpins.