Discovery of a Vascular Endothelial Stem Cell (VESC) Population Required for Vascular Regeneration and Tissue Maintenance
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Chapter 20 *Lecture Powerpoint the Circulatory System: Blood Vessels and Circulation
Chapter 20 *Lecture PowerPoint The Circulatory System: Blood Vessels and Circulation *See separate FlexArt PowerPoint slides for all figures and tables preinserted into PowerPoint without notes. Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Introduction • The route taken by the blood after it leaves the heart was a point of much confusion for many centuries – Chinese emperor Huang Ti (2697–2597 BC) believed that blood flowed in a complete circuit around the body and back to the heart – Roman physician Galen (129–c. 199) thought blood flowed back and forth like air; the liver created blood out of nutrients and organs consumed it – English physician William Harvey (1578–1657) did experimentation on circulation in snakes; birth of experimental physiology – After microscope was invented, blood and capillaries were discovered by van Leeuwenhoek and Malpighi 20-2 General Anatomy of the Blood Vessels • Expected Learning Outcomes – Describe the structure of a blood vessel. – Describe the different types of arteries, capillaries, and veins. – Trace the general route usually taken by the blood from the heart and back again. – Describe some variations on this route. 20-3 General Anatomy of the Blood Vessels Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Capillaries Artery: Tunica interna Tunica media Tunica externa Nerve Vein Figure 20.1a (a) 1 mm © The McGraw-Hill Companies, Inc./Dennis Strete, photographer • Arteries carry blood away from heart • Veins -

The Circulatory System
TheThe CirculatoryCirculatory SystemSystem Xue Hui DepartmentDepartment ofof HistologyHistology && EmbryologyEmbryology TheThe CirculatoryCirculatory SystemSystem Cardiovascular system (blood vascular system) Heart Artery Capillary Vein Lymphatic vascular system Lymphatic capillary Lymphatic vessel Lymphatic duct II GeneralGeneral structurestructure ofof thethe bloodblood vesselsvessels Tunica intima Tunica media Tunica adventitia Drawing of a medium-sized muscular artery, showing its layers. II GeneralGeneral structurestructure ofof thethe bloodblood vesselsvessels IIII ArteryArtery Large artery Medium-sized artery Small artery Arteriole II Artery LargeLarge arteryartery Structure Tunica intima Tunica media 40-70 layers of elastic lamina Smooth muscle cells, collagenous fibers Tunica adventitia Function Carry the blood from the heart to the middle arteries Tunica Tunica intima intima Tunica Tunica media media Tunica Tunica adventitia adventitia Transverse sections showing part of a large elastic artery showing a well developed tunica media containing several elastic laminas. II Artery MediumMedium--sizedsized arteryartery Structure Tunica intima: clear internal elastic membrane Tunica media: 10-40 layers of smooth muscle cells Tunica adventitia: external elastic membrane Function Regulate the distribution of the blood to various parts of the body Tunica Internal intima elastic membrane Tunica media External elastic membrane Tunica adventitia II Artery SmallSmall arteryartery Structure characteristic Diameter:0.3-1mm Tunica intima: clear -

Anatomy Review: Blood Vessel Structure & Function
Anatomy Review: Blood Vessel Structure & Function Graphics are used with permission of: Pearson Education Inc., publishing as Benjamin Cummings (http://www.aw-bc.com) Page 1. Introduction • The blood vessels of the body form a closed delivery system that begins and ends at the heart. Page 2. Goals • To describe the general structure of blood vessel walls. • To compare and contrast the types of blood vessels. • To relate the blood pressure in the various parts of the vascular system to differences in blood vessel structure. Page 3. General Structure of Blood Vessel Walls • All blood vessels, except the very smallest, have three distinct layers or tunics. The tunics surround the central blood-containing space - the lumen. 1. Tunica Intima (Tunica Interna) - The innermost tunic. It is in intimate contact with the blood in the lumen. It includes the endothelium that lines the lumen of all vessels, forming a smooth, friction reducing lining. 2. Tunica Media - The middle layer. Consists mostly of circularly-arranged smooth muscle cells and sheets of elastin. The muscle cells contract and relax, whereas the elastin allows vessels to stretch and recoil. 3. Tunica Adventitia (Tunica Externa) - The outermost layer. Composed of loosely woven collagen fibers that protect the blood vessel and anchor it to surrounding structures. Page 4. Comparison of Arteries, Capillaries, and Veins • Let's compare and contrast the three types of blood vessels: arteries, capillaries, and veins. • Label the artery, capillary and vein. Also label the layers of each. • Arteries are vessels that transport blood away from the heart. Because they are exposed to the highest pressures of any vessels, they have the thickest tunica media. -

Blood Vessels: Part A
Chapter 19 The Cardiovascular System: Blood Vessels: Part A Blood Vessels • Delivery system of dynamic structures that begins and ends at heart – Arteries: carry blood away from heart; oxygenated except for pulmonary circulation and umbilical vessels of fetus – Capillaries: contact tissue cells; directly serve cellular needs – Veins: carry blood toward heart Structure of Blood Vessel Walls • Lumen – Central blood-containing space • Three wall layers in arteries and veins – Tunica intima, tunica media, and tunica externa • Capillaries – Endothelium with sparse basal lamina Tunics • Tunica intima – Endothelium lines lumen of all vessels • Continuous with endocardium • Slick surface reduces friction – Subendothelial layer in vessels larger than 1 mm; connective tissue basement membrane Tunics • Tunica media – Smooth muscle and sheets of elastin – Sympathetic vasomotor nerve fibers control vasoconstriction and vasodilation of vessels • Influence blood flow and blood pressure Tunics • Tunica externa (tunica adventitia) – Collagen fibers protect and reinforce; anchor to surrounding structures – Contains nerve fibers, lymphatic vessels – Vasa vasorum of larger vessels nourishes external layer Blood Vessels • Vessels vary in length, diameter, wall thickness, tissue makeup • See figure 19.2 for interaction with lymphatic vessels Arterial System: Elastic Arteries • Large thick-walled arteries with elastin in all three tunics • Aorta and its major branches • Large lumen offers low resistance • Inactive in vasoconstriction • Act as pressure reservoirs—expand -

BIO 2402 Resource 8
BIO 2402 — Human Anatomy & Physiology Week 8: March 7-13 This week we will be covering the last chapter of the cardiovascular system about blood vessels. You should be able to know the different types of blood vessels, the factors that influence blood pressure, and the difference between baroreceptors and chemoreceptors. Remember that the Tutoring Center offers free individual and group tutoring for this class. Our Group Tutoring sessions will be every Wednesday from 6:00-7:00 PM CST. You can reserve a spot at https://baylor.edu/tutoring. KEY TERM: Vasoconstrictor, Vasodilator, Elastic Artery, Fenestrated Capillary, Vasomotor Center Blood vessel tunics & tissues Arteries Elastic/conducting: large diameter, thick walls, elastic tissue Aorta, brachiocephalic, common carotids, subclavian, common iliac Muscular/distributing: trans. blood to arterioles, thickest tunica media, less elastic tissue Brachials, mesenterics, femorals, tibials Arterioles: only smooth muscle & endothelium Metarterioles: smaller arterioles, precapillary sphincters regulate blood flow into the capillaries. Capillaries Fenestrated: small pores that material can pass through Sinusoids: type of fenestrated capillary in the liver, spleen, pancreas, and bone marrow Continuous: more common, lacks fenestrations All of these arteries and veins can be visualized using the diagram above. Blood pressures For blood pressure we will focus on capillary dynamics and factors affecting vessel diameter. All images are taken from Dr. Taylor’s textbook Vasoconstrictors & vasodilators are factors -

Blood and Lymph Vascular Systems
BLOOD AND LYMPH VASCULAR SYSTEMS BLOOD TRANSFUSIONS Objectives Functions of vessels Layers in vascular walls Classification of vessels Components of vascular walls Control of blood flow in microvasculature Variation in microvasculature Blood barriers Lymphatic system Introduction Multicellular Organisms Need 3 Mechanisms --------------------------------------------------------------- 1. Distribute oxygen, nutrients, and hormones CARDIOVASCULAR SYSTEM 2. Collect waste 3. Transport waste to excretory organs CARDIOVASCULAR SYSTEM Cardiovascular System Component function Heart - Produce blood pressure (systole) Elastic arteries - Conduct blood and maintain pressure during diastole Muscular arteries - Distribute blood, maintain pressure Arterioles - Peripheral resistance and distribute blood Capillaries - Exchange nutrients and waste Venules - Collect blood from capillaries (Edema) Veins - Transmit blood to large veins Reservoir Larger veins - receive lymph and return blood to Heart, blood reservoir Cardiovascular System Heart produces blood pressure (systole) ARTERIOLES – PERIPHERAL RESISTANCE Vessels are structurally adapted to physical and metabolic requirements. Vessels are structurally adapted to physical and metabolic requirements. Cardiovascular System Elastic arteries- conduct blood and maintain pressure during diastole Cardiovascular System Muscular Arteries - distribute blood, maintain pressure Arterioles - peripheral resistance and distribute blood Capillaries - exchange nutrients and waste Venules - collect blood from capillaries -

Microscopic Anatomy of the Human Vertebro-Basilar System
MICROSCOPIC ANATOMY OF THE HUMAN VERTEBRO-BASILAR SYSTEM RENATO P. CHOPARD * — GUILHERME DE ARAÚJO LUCAS * ADRIANA LAUDANA* SUMMARY — Concerning the structure of connective-muscular components the authors stu died the walls of the terminal segments of the vertebral arteries as well as the basilar artery, utilizing the following staining methods: Azan modified by Heideinheim, Weigert's resor-¬ cin-fuchsin, and Weigert modified by van Gieson. It was established that wall of the verte-¬ bro-basilar system exhibits a mixed structure, muscular and elastic, by means of which the vessels are adjusted to the specific blood circulation conditions. Thus, vertebral arteries show in the most external layer of tunica media an evident external elastic lamina. In contrast, in the basilar artery the elastic tissue is localized mainly in the tunica media, and is distributed heterogeneously. In its caudal segment the elastic fibers are situated in the most internal layer of tunica media, and in the cranial segment the elastic component is homogenously distributed in the whole of tunica media. Anatomia microscópica do sistema, vértebro basilar no homem. RESUMO — Os autores estudam a disposição dos componentes fibromusculare» da parede das artérias vertebrais em seus segmentos terminais e a artéria basilar, usando os seguintes métodos de coloração: Azan modificado por Heidenheim, resorcina-fucsina de Weigert e Weigert modificado por van Gieson. O sistema vértebro-basilar apresentou em sua parede estrutura mista, muscular e elástica, pela qual os vasos se adaptam às condições circulató rias especificas daquela região. Besta forma, ias artérias vertebrais apresentam na camada mais externa da túnica média uma lâmina elástica limitante externa evidente, ao contrário do encontrado na artéria basilar. -

Morphometric Analysis of Thoracic Aorta in Slc39a13/Zip13-KO Mice
Cell and Tissue Research (2019) 376:137–141 https://doi.org/10.1007/s00441-018-2977-9 SHORT COMMUNICATION Morphometric analysis of thoracic aorta in Slc39a13/Zip13-KO mice Takuya Hirose1 & Takamasa Shimazaki1 & Naoki Takahashi1 & Toshiyuki Fukada2 & Takafumi Watanabe1,3 & Prasarn Tangkawattana 1,4 & Kazushige Takehana1 Received: 18 July 2018 /Accepted: 13 October 2018 /Published online: 4 January 2019 # Springer-Verlag GmbH Germany, part of Springer Nature 2019 Abstract Ehlers–Danlos syndrome (EDS) is a collection of inheritable diseases involving the musculoskeletal, integumentary and visual systems. Spondylodysplastic EDS-ZIP13 (spEDS-ZIP13: OMIM 612350) was recently defined as a new form of EDS. Although vasculitis has been found in many spEDS-ZIP13 patients, vascular pathology has not been included as a pathognomonic lesion of this type of EDS. We investigate the morphometry of the thoracic aorta in wild-type and Zip13-knockout (Zip13-KO) mice. Our assessment found abnormalities in the number and morphology of elastic and cellular components in the aortic wall, especially the tunica media, of Zip13-KO mice, indicating aortic fragility. Accordingly, our major findings (vascular smooth muscle cells with small nuclei, small percentage of elastic membrane area per tunica media, many large elastic flaps) should be considered vulnerable characteristics indicating fragility of the aorta in patients with spEDS-ZIP13. Keywords Aorta . Elasticity . Elastic membrane . Morphometry . Slc39a13/Zip13-KO mouse Introduction (Fukada et al. 2008). ZIP13, a member of the SLC39/ZIP family, is an intercellular zinc transporter located in the Ehlers–Danlos syndrome (EDS) is a collection of hereditary Golgi complex of osteoblasts, chondrocytes, pulpal cells diseases that manifest as skin hyperextension, joint hypermo- and fibroblasts (Fukada et al. -

The Circulatory System
The Circulatory System Lec.10 Histology 1 The Circulatory System includes both 1. blood vascular system 2. lymphatic vascular system 2 blood vascular system 1. The heart, an organ whose function is to pump the blood. 2. The arteries, a series of efferent vessels that become smaller as they branch, and whose function is to carry the blood, with its nutrients and oxygen, to the tissues. 3. The capillaries, the smallest blood vessels, constituting a complex network of thin tubules that branch profusely in almost every organ and through whose walls the interchange between blood and tissues takes place. 4. The veins, which result from the convergence of capillaries into a system of larger channels that continue enlarging as they approach the heart, toward which they convey the blood to be pumped again. 3 The lymphatic vascular system • The lymphatic vascular system, begins with the lymphatic capillaries, which are closed-ended tubules that merge to form vessels of steadily increasing size; these vessels terminate in the blood vascular system emptying into the large veins near the heart. • One of the functions of the lymphatic system is to return the fluid of the tissue spaces to the blood. • The internal surface of all components of the blood and lymphatic systems is lined by a single layer of a squamous epithelium, called endothelium. 4 Heart • The heart is a muscular organ that contracts rhythmically, pumping the blood through the circulatory system . • The right and left ventricles pump blood to the lungs and the rest of the body respectively; right and left atria receive blood from the body and the pulmonary veins respectively. -

Almondi Fagundes.Pmd
Fagundes Efeitos da remoção da túnica adventícia da aorta descendente em suínos Artigo Original133 Efeitos da remoção da túnica adventícia da aorta descendente em suínos Effects of removal of the adventitia of the descending aorta and structural alterations in the tunica media in pigs ALMONDI FAGUNDES1; ADAMASTOR HUMBERTO PEREIRA, TCBC-RS2; ROSE KARINA CORRÊA3; MARÍLIA TERESA DE OLIVEIRA4; RUBENS RODRIGUEZ5 RESUMO Objetivo: Investigar os efeitos da remoção da adventícia da aorta em suínos. Métodos: O experimento foi realizado com oito suínos. Removeu-se a camada adventícia da aorta descendente. Após a eutanásia com duas, quatro, seis e oito semanas, o segmento aórtico era removido. Após, eram feitos cortes histológicos com a coloração de hematoxilina e eosina (HE) e pelo método de Weigert – Van Gieson. Resultados: Após duas semanas identificou-se um leve desarranjo do terço externo da túnica média. Nos animais sacrificados após quatro semanas observou-se um desarranjo estrutural dos terços externos da túnica média. Após seis semanas observou-se necrose da parede aórtica. Finalmente, após oito semanas além da fibrose da parede aórtica identificou-se a destruição da lâmina elástica interna. Conclusão: A remoção da adventícia da aorta em suínos levou à alterações degenerativas da média, determinando perda da estrutura da parede aórtica que é variável em sua localização, intensidade e forma, dependendo do tempo a partir do qual se estabeleceu a lesão isquêmica. Descritores: Aorta. Vasa vasorum. Microcirculação. Isquemia. Dissecação. INTRODUÇÃO semanas de interrupção do fluxo sanguíneo através do VV da aorta descendente em modelo suíno. dissecção da aorta é a catástrofe mais devastadora A das doenças cardiovasculares, mas o mecanismo pelo qual a dissecção ocorre ainda não é totalmente entendido. -
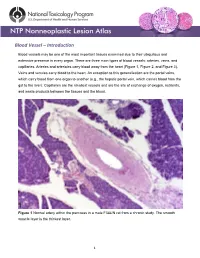
Blood Vessel – Introduction
Blood Vessel – Introduction Blood vessels may be one of the most important tissues examined due to their ubiquitous and extensive presence in every organ. There are three main types of blood vessels: arteries, veins, and capillaries. Arteries and arterioles carry blood away from the heart (Figure 1, Figure 2, and Figure 3). Veins and venules carry blood to the heart. An exception to this generalization are the portal veins, which carry blood from one organ to another (e.g., the hepatic portal vein, which carries blood from the gut to the liver). Capillaries are the smallest vessels and are the site of exchange of oxygen, nutrients, and waste products between the tissues and the blood. Figure 1 Normal artery within the pancreas in a male F344/N rat from a chronic study. The smooth muscle layer is the thickest layer. 1 Blood Vessel – Introduction Figure 2 Normal tunica media (asterisks) and tunica intima (arrows) of an artery within the pancreas in a male F344/N rat from a chronic study (higher magnification of Figure 1). Arteries and veins have walls composed of three layers: the tunica intima, the tunica media, and the tunica adventitia. The tunica intima is composed of endothelial cells on a basement membrane and a subendothelial layer of collagen and elastic fibers. The tunica media consists of smooth muscle cells, elastic fibers, and collagen. The tunica adventitia is composed of collagen and elastic fibers. The relative amounts of each of these components vary depending on the type of vessel. The tunica intima and tunica media of elastic arteries are generally thicker than those of the other types, and the tunica 2 Blood Vessel – Introduction Figure 3 Normal aorta in a male B6C3F1/N mouse from a chronic study. -

Blood Vessel, Artery – Hypertrophy, Medial
Blood Vessel, Artery – Hypertrophy, Medial Figure Legend: Figure 1 Lung, Artery - Hypertrophy, Medial in a male B6C3F1/N mouse from a subchronic study. The tunica media of two small muscular arteries is thickened. Figure 2 Lung, Artery - Hypertrophy, Medial in a male B6C3F1/N mouse from a subchronic study (higher magnification of Figure 1). Medial hypertrophy of two small muscular arteries is apparent; note the lack of inflammation. Comment: Arterial medial hypertrophy is characterized by circumferential thickening of the tunica media of arteries (Figure 1 and Figure 2) that often results in narrowing of the vascular lumen. Thickening is caused by concentric layers of hyperplastic and hypertrophied smooth muscle cells, along with increases in collagen, elastin, glycosaminoglycans, electrolytes, and water. Although increases in connective tissue of the tunica adventitia may also be noted, the predominant finding is smooth muscle cell hypertrophy and proliferation. Medial hypertrophy is most often noted as a secondary response to hypertension from various etiologies (systemic or associated with chronic renal disease) and occurs in both large and small muscular arteries. The lesion is uncommon as a spontaneous finding in laboratory rats, even in the case of severe chronic progressive nephropathy. It may occur occasionally in overloaded collateral arteries adjacent to areas of vascular occlusion. The lesion is most important and most commonly seen in hypertensive rat strains (SHR) and in other created models of hypertension. Induction of hypertension in rats has been achieved through various means, including chronic hypoxia, hyperoxia, and monocrotaline injection. Decreased kidney function through radical nephrectomy or arterial restriction also results in hypertension and medial hypertrophy 1 Blood Vessel, Artery – Hypertrophy, Medial in rats.