REVISION 1 Intra-Eruptive Trachyte
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Mineralogy and Chemistry of the Anorogenic Tertiary Silicic Volcanics
JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 86, NO. Bll, PAGES 10242-10256, NOVEMBER 10, 1981 The Mineralogyand Chemistryof the AnorogenicTertiary SilicicVolcanics of S.E. Queenslandand N.E. New South Wales, Australia A. EWART Departmentof Geology& Mineralogy,University of Queensland,St. Lucia,Brisbane, Queensland 4067 The Late Oligocene-EarlyMiocene volcanismof this regionis chemicallystrongly bimodal; the mafic lavas(volmetrically dominant) comprise basalts, hawaiites, and tholeiiticandesites, while the silicic eruptivesare mainly comendites,potassic trachytes, and potassic,high-silica rhyolites.The comendites and rhyoliteshave distinctivetrace element abundancepatterns, notably the extreme depletionsof Sr, Ba, Mg, Mn, P, Cr, V, and Eu, and the variable em'ichraentof suchelements as Rb, Zr, Pb, Nb, Zn, U, and Th. The trachytesexhibit thesecharacteristics to lesserdegrees. The comenditesare distinguished from the rhyolitesby their overall relative enrichmentof the more highly chargedcations (e.g., LREE, Nb, Y, and especiallyZr) and Zn. The phenocrystmineralogy of the trachytesand rhyolitescomprises various combinationsof the following phases:sodic plagioclase(albite-andesine), calcic anorthoclase, sanidine, quartz, ferroaugite-ferrohedenbergite,ferrohypersthene, fayalitic olivine, ilmenite, titano- magnetite,and rarely biotite (near annite) and Fe-hastingsiticamphibole. Accessories include apatite, zircon, chevkinite (ferrohedenbergite-bearingrhyolites only), and allanite (amphibole and botite rhyo- lites only). The comenditesgenerally contain -

Source to Surface Model of Monogenetic Volcanism: a Critical Review
Downloaded from http://sp.lyellcollection.org/ by guest on September 28, 2021 Source to surface model of monogenetic volcanism: a critical review I. E. M. SMITH1 &K.NE´ METH2* 1School of Environment, University of Auckland, Auckland, New Zealand 2Volcanic Risk Solutions, Massey University, Palmerston North 4442, New Zealand *Correspondence: [email protected] Abstract: Small-scale volcanic systems are the most widespread type of volcanism on Earth and occur in all of the main tectonic settings. Most commonly, these systems erupt basaltic magmas within a wide compositional range from strongly silica undersaturated to saturated and oversatu- rated; less commonly, the spectrum includes more siliceous compositions. Small-scale volcanic systems are commonly monogenetic in the sense that they are represented at the Earth’s surface by fields of small volcanoes, each the product of a temporally restricted eruption of a composition- ally distinct batch of magma, and this is in contrast to polygenetic systems characterized by rela- tively large edifices built by multiple eruptions over longer periods of time involving magmas with diverse origins. Eruption styles of small-scale volcanoes range from pyroclastic to effusive, and are strongly controlled by the relative influence of the characteristics of the magmatic system and the surface environment. Gold Open Access: This article is published under the terms of the CC-BY 3.0 license. Small-scale basaltic magmatic systems characteris- hazards associated with eruptions, and this is tically occur at the Earth’s surface as fields of small particularly true where volcanic fields are in close monogenetic volcanoes. These volcanoes are the proximity to population centres. -

31 a Preliminary Study Of
31 A PRELIMINARY STUDY OF THE: TERTIARY VOLCANIC AND SEDIMENTARY ROCKS, GÜMELE, ESKİŞEHİR Eskişehir, Gümele Çevresindeki Tersiyer Volkanik ve Sedimanter Kayaçlarda Bir Ön Çalışma Taylan Lünel Middle East Technical University, Department of Geological Engineering Ankara ÖZ. — Seyitgazi-Eskişehir antiklinoriumu'nun çok fazla deforme olmuş ve metamorfizmaya uğramış kayaçlarının kuzey-kuzeybatısında bulunan sedimanter ve volkanik kayaçlar incelenmiştir. Karasal ve gölsel fasiyesde meydana gelen Tersi- yer sedimanter kayaçlar Güney Eskişehir küvetinde olunmuşlardır. Karasal fasiye- si meydana getiren kayaç birimlerini kaba kumtaşları, kumtaşları, bitki kalıntıları ihtiva eden kil ve marnlar ve serpantinit blokları taşıyan bazal konglomerası teşkil etmektedir. Gölsel fasiyes ise genellikle killi ve tüflü kalkerler, kalkerler, marnlar, kon- glomeralar ve tüflerden meydana gelmiştir. Küvetteki en eski sedimanlar ve piroklas- tikler Alt Miosen'de oluşmuşlardır. Yataya yakın konumlanmış bazik-intermediyar lav akıntıları Pliosen yaşlı olup Altüst Neojen sedimantasyon kesikliğinde meyda- na gelmiştir. Üst Neojen sedimanter kayaçları intermediyar-basaltik volkaniklerin üzerinde ince bandlar şeklinde bulunurlar. Bu birim marn ve kalkerlerden meyda- na gelmiştir. Alt Miosen’de asid volkanik faaliyetler neticesinde meydana gelen sil- lar (unweldd tuffs) oligomikt konglomeralardan evvel teşekkül etmiştir. Bu volkanik aktitivite muhtemelen kesikli ve kısıtlı olarak devam etmiş ve tüflü kalkerleri mey- dana getirmiştir. Pliosen yaşlı bazik-intermediyar -

Geological Mapping, Structural Setting and Petrographic Description of the Archean Volcanic Rocks of Mnanka Area, North Mara
PROCEEDINGS, 43rd Workshop on Geothermal Reservoir Engineering Stanford University, Stanford, California, February 12-14, 2018 SGP-TR-213 Geological Mapping, Structural Setting and Petrographic Description of the Archean Volcanic Rocks of Mnanka Area, North Mara Ezra Kavana Acacia Mining PLc, North Mara Gold Mine, Department of Geology, P. O. Box 75864, Dar es Salaam, Tanzania Email: [email protected] Keywords: Musoma Mara Greenstone Belt, Mnanka volcanics, Archaean rocks and lithology ABSTRACT The Mnanka area is situated within the Musoma Mara Greenstone Belt, the area is near to Nyabigena, Gokona and Nyabirama gold mines. Mnanka area comprises of the sequence of predominant rhyolitic volcanic rocks, chert and metasediments. Gold mineralizations in Mnanka area is structure controlled and occur mainly as hydrothermal disseminated intrusion related deposits. Hence the predominant observed structures are joints and flow banding. Measurements from flow banding plotted on stereonets using win-TENSOR software has provided an estimate for the general strike of the area lying 070° to 100° dipping at an average range angle of 70° to 85° while data from joints plotted on stereonets suggest multiple deformation events one of which conforms to the East Africa Rift System (striking WSW-ENE, NNE-SSW and N-S). 1. INTRODUCTION This paper focuses on performing a systematic geological mapping and description of structures and rocks of the Mnanka area. The Mnanka area is located in the Mara region, Tarime district within the Musoma Mara Greenstone Belt. The gold at Mnanka is host ed by volcanic rocks that belong to the Musoma Mara Greenstone Belt (Figure 1). The Mnanka volcanics are found within the Kemambo group that comprises of the sequence of predominant rhyolitic volcanic rocks, chert and metasediments south of the Nyarwana fault. -

Chemical and Isotopic Studies of Monogenetic Volcanic Fields: Implications for Petrogenesis and Mantle Source Heterogeneity
MIAMI UNIVERSITY The Graduate School Certificate for Approving the Dissertation We hereby approve the Dissertation of Christine Rasoazanamparany Candidate for the Degree DOCTOR OF PHILOSOPHY ______________________________________ Elisabeth Widom, Director ______________________________________ William K. Hart, Reader ______________________________________ Mike R. Brudzinski, Reader ______________________________________ Marie-Noelle Guilbaud, Reader ______________________________________ Hong Wang, Graduate School Representative ABSTRACT CHEMICAL AND ISOTOPIC STUDIES OF MONOGENETIC VOLCANIC FIELDS: IMPLICATIONS FOR PETROGENESIS AND MANTLE SOURCE HETEROGENEITY by Christine Rasoazanamparany The primary goal of this dissertation was to investigate the petrogenetic processes operating in young, monogenetic volcanic systems in diverse tectonic settings, through detailed field studies, elemental analysis, and Sr-Nd-Pb-Hf-Os-O isotopic compositions. The targeted study areas include the Lunar Crater Volcanic Field, Nevada, an area of relatively recent volcanism within the Basin and Range province; and the Michoacán and Sierra Chichinautzin Volcanic Fields in the Trans-Mexican Volcanic Belt, which are linked to modern subduction. In these studies, key questions include (1) the role of crustal assimilation vs. mantle source enrichment in producing chemical and isotopic heterogeneity in the eruptive products, (2) the origin of the mantle heterogeneity, and (3) the cause of spatial-temporal variability in the sources of magmatism. In all three studies it was shown that there is significant compositional variability within individual volcanoes and/or across the volcanic field that cannot be attributed to assimilation of crust during magmatic differentiation, but instead is attributed to mantle source heterogeneity. In the first study, which focused on the Lunar Crater Volcanic Field, it was further shown that the mantle heterogeneity is formed by ancient crustal recycling plus contribution from hydrous fluid related to subsequent subduction. -

Relationships Between Pre-Eruptive Conditions and Eruptive Styles of Phonolite-Trachyte Magmas Joan Andújar, Bruno Scaillet
Relationships between pre-eruptive conditions and eruptive styles of phonolite-trachyte magmas Joan Andújar, Bruno Scaillet To cite this version: Joan Andújar, Bruno Scaillet. Relationships between pre-eruptive conditions and eruptive styles of phonolite-trachyte magmas. Lithos, Elsevier, 2012, 152 (1), pp.122-131. 10.1016/j.lithos.2012.05.009. insu-00705854 HAL Id: insu-00705854 https://hal-insu.archives-ouvertes.fr/insu-00705854 Submitted on 10 Jul 2012 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. 1 Relationships between pre-eruptive conditions and eruptive styles of phonolite-trachyte magmas JOAN ANDÚJAR*,a AND BRUNO SCAILLETa a. CNRS/INSU-UNIVERSITÉ D’ORLÉANS-BRGM ; INSTITUT DES SCIENCES DE LA TERRE D’ORLEANS, UMR 6113 - 1A, RUE DE LA FÉRROLLERIE-45071 ORLEANS CEDEX 2 (FRANCE) * Corresponding author : Joan Andújar phone number : (+33) 2 38 25 53 87 Fax: (+33) 02 38 63 64 88 e-mail address: [email protected] Bruno Scaillet e-mail address: [email protected] KEY WORDS: Phase equilibria, phonolite, trachyte, experimental petrology, eruptive dynamic, explosive, effusive, andesite, rhyolite, melt viscosity, magma viscosity. 2 Abstract Phonolitic eruptions can erupt either effusively or explosively, and in some cases develop highly energetic events such as caldera-forming eruptions. -

THE HAWAIIAN-EMPEROR VOLCANIC CHAIN Part I Geologic Evolution
VOLCANISM IN HAWAII Chapter 1 - .-............,. THE HAWAIIAN-EMPEROR VOLCANIC CHAIN Part I Geologic Evolution By David A. Clague and G. Brent Dalrymple ABSTRACT chain, the near-fixity of the hot spot, the chemistry and timing of The Hawaiian-Emperor volcanic chain stretches nearly the eruptions from individual volcanoes, and the detailed geom 6,000 km across the North Pacific Ocean and consists of at least etry of volcanism. None of the geophysical hypotheses pro t 07 individual volcanoes with a total volume of about 1 million posed to date are fully satisfactory. However, the existence of km3• The chain is age progressive with still-active volcanoes at the Hawaiian ewell suggests that hot spots are indeed hot. In the southeast end and 80-75-Ma volcanoes at the northwest addition, both geophysical and geochemical hypotheses suggest end. The bend between the Hawaiian and .Emperor Chains that primitive undegassed mantle material ascends beneath reflects a major change in Pacific plate motion at 43.1 ± 1.4 Ma Hawaii. Petrologic models suggest that this primitive material and probably was caused by collision of the Indian subcontinent reacts with the ocean lithosphere to produce the compositional into Eurasia and the resulting reorganization of oceanic spread range of Hawaiian lava. ing centers and initiation of subduction zones in the western Pacific. The volcanoes of the chain were erupted onto the floor of the Pacific Ocean without regard for the age or preexisting INTRODUCTION structure of the ocean crust. Hawaiian volcanoes erupt lava of distinct chemical com The Hawaiian Islands; the seamounts, hanks, and islands of positions during four major stages in their evolution and the Hawaiian Ridge; and the chain of Emperor Seamounts form an growth. -

Rhyolite and Trachyte Formation at Lake City Caldera: Insight from Quantitative Textural and Geochemical Analyses
Michigan Technological University Digital Commons @ Michigan Tech Dissertations, Master's Theses and Master's Reports 2016 Rhyolite and Trachyte Formation at Lake City Caldera: Insight from Quantitative Textural and Geochemical Analyses Jordan Lubbers Michigan Technological University, [email protected] Copyright 2016 Jordan Lubbers Recommended Citation Lubbers, Jordan, "Rhyolite and Trachyte Formation at Lake City Caldera: Insight from Quantitative Textural and Geochemical Analyses", Open Access Master's Thesis, Michigan Technological University, 2016. https://doi.org/10.37099/mtu.dc.etdr/99 Follow this and additional works at: https://digitalcommons.mtu.edu/etdr Part of the Geochemistry Commons, and the Geology Commons RHYOLITE AND TRACHYTE FORMATION AT LAKE CITY CALDERA: INSIGHT FROM QUANTITATIVE TEXTURAL AND GEOCHEMICAL ANALYSES By Jordan E. Lubbers A THESIS Submitted in partial fulfillment of the requirements for the degree of MASTER OF SCIENCE In Geology MICHIGAN TECHNOLOGICAL UNIVERSITY 2016 © 2016 Jordan E. Lubbers This thesis has been approved in partial fulfillment of the requirements for the Degree of MASTER OF SCIENCE in Geology. Geological and Mining Engineering and Sciences ThesisDepartment Advisor: ofChad Deering Committee Member: Olivier Bachmann Committee Member: William Rose Department Chair: John Gierke Table of Contents Acknowledgements ................................................................................................................................................. 6 Abstract ...................................................................................................................................................................... -
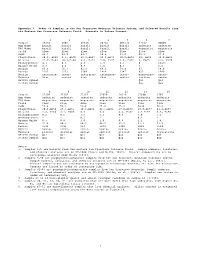
1 Appendix 3. Modes of Samples in the San Francisco Mountain
Appendix 3. Modes of Samples in the San Francisco Mountain Volcanic System, and Selected Basalts from the Eastern San Francisco Volcanic Field. Minerals in Volume Percent. 1 2 3 4 5 6 7 Sample 3823A 2A06 2812A 2031A 2031.B 3732J DC04B Map Name basalt basalt basalt basalt basalt andesite andesite TAS Name basalt basalt basalt basalt basalt mugearite mugearite Field flow flow flow flow flow flow flow SiO2 48.7 48.9 49.7 50.4 51.0 52.2 53.0 Plagioclase 14.6, An69 8.3, An70 25.6,An67 25.5,An71 20.2,An74 29, An64 32.0,An62 Olivine 11.2, Fo83 10.9,Fo84 8.1, Fo73 7.0, Fo77 3.9, Fo76 3, Fo75 6.6, Fo75 Clinopyroxene 4.3 4.3 4.6 1.8 1.3 0 trace Opaque Oxide 4.1 0.2 0.5 1.6 0.3 1 2.4 Matrix 65.8 76.3 61.2 64.1 74.4 67 59.0 Points 1165 1173 1504 1464 1347 827 1549 Matrix intergran- inter- intergran- intergran- inter- microcrys- inter- Texture ular sertal ular ular sertal talline sertal MF1959 Symbol Qa1 Qa1 I-1663 Symbol Qao Qao 8 9 10 11 12 13 14 Sample 3732K 3732Z 3732Y 2705D 3831A 3729Q 3707 Map Name andesite andesite andesite andesite andesite andesite andesite TAS Name mugearite mugearite mugearite mugearite mugearite mugearite mugearite Field flow flow dike flow flow flow flow SiO2 53.4 53.7 53.8 55.2 55.3 56.0 56.6 Plagioclase 24.8,An64 15.3,An61 30.2,An61 42.1,An52 27.0,An62 29.8,An57 41.2,An57 Olivine 2.8, Fo69 3.1, Fo65 4.1 5.6 4.3, Fo62 3.7 Fo57 2.5, Fo58 Clinopyroxene 0.2 0.5 1.5 2.1 0.8 3.0 1.1 Orthopyroxene - - 0.5 - - 0.2 - Opaque Oxide 0.3 0.8 3.0 1.0 0.6 2.1 1.4 Matrix 71.8 80.3 60.7 49.2 67.2 61.1 53.8 Points 1196 1785 854 1844 1428 1029 1540 Matrix hyalo- inter- inter- hyalo- hyalo- hyalo- micro- Texture pilitic granular sertal ophitic pilitic ophitic crystalline MF1959 Symbol Qa1 Qa1 Qai Qa1 Qa2 Qa2 Qa2 I-1663 Symbol Qao Qao Qai Qao Qay Qay Qay Notes: 1. -

The Jackson Volcano
THE DEPARTMENT OF ENVIRONMENTAL QUALITY Office of Geology P. 0. Box 20307 Volume 18, Number 3 Jackson, Mississippi 39289-1307 September 1997 THE JACKSON VOLCANO David T. Dockery ID, John C. Marble, and Jack Henderson Mississippi Office of Geology INTRODUCTION One of the most interesting geologic features ofMississippi is the Jackson Volcano, which rests only 2900 feet beneath the state's capital city. The volcano's dense core forms one ofthe most prominent structural anomalies found on gravity and magnetic surveys of the state, showing tightly wrapped con tours of increasing gravity and magnetic deflection like a crowded bull's- eye (Figure l ). This igneous complex and the up Iifted formations around it comprise a structure known as the Jackson Dome. Northwest ofthe Jackson Volcano in southern Humphreys County is the Midnight Volcano and the associ ated volcanic terrain ofthe Sharkey Platform . Dense igneous rock below Jackson and Midnight increase the force ofgravity slightly as shown in Figure I. A person tipping the scales at Jackson or Midnight would be slightly heavier than elsewhere in the state (if anyone would like to use that for an excuse). No other capital city or major population center is situated above an extinct volcano, even though the recent movie "Volcano" fictitiously placed Los Angeles, California, above one. Monroe, Louisiana, is Jackson 's sister city in being a close second, as it rests above a volcanic terrain known as the Monroe Uplift. However, the position ofJackson's downtown district above the throat of an extinct Cretaceous volcano seems to be unique. Ifthe Jackson Volcano were to ever vent itself in the future, the Coliseum would be near ground zero. -

Direct Derivation of Benmoreite to Phonolite
EXPERIMENTAL DERIVATION OF NEPHELINE SYENITE AND PHONOLITE LIQUIDS BY PARTIAL MELTING OF UPPER MANTLE PERIDOTITES Didier LAPORTE, Sarah LAMBART, Pierre SCHIANO, and Luisa OTTOLINI SUPPLEMENTARY MATERIAL 1. Experimental and analytical techniques 1.1 Starting materials. The starting material used to prepare our two fertile mantle compositions was a fresh spinel lherzolite xenolith (Bri3) from Mont Briançon volcano, French Massif Central. Because a fine grain size is critical to attain chemical equilibrium, lherzolite Bri3 was first pulverized in a micronizing mill for 30 min to reduce its grain size to 2-4 µm. The powder was then fired 5 hours at 900°C in a CO2/H2 atmosphere with gas flow rates adjusted to yield an oxygen fugacity between the magnetite-wüstite and the iron-wüstite -15.91 buffers (fO2 = 10 bar). As Bri3 contains only 100 ppm K2O, small amounts of synthetic basalt B2 were added to Bri3 to prepare the fertile mantle compositions MBK (410 ppm K2O) and MBK+ (930 ppm K2O; compositions Bri3, B2, MBK and MBK+ are given in Table 1). Mixtures of Bri3 and B2 were homogenized by grinding in an agate mortar for one hour. The starting material for reversal experiments MBK+13 and MBK+14 was a mixture of 80.16 % MBK+ and 19.84 % phonolite gel +6 (Table 1). Phonolite gel +6 was prepared using the gel method (Luth and Ingamells, 1965), and then fired at the same T and fO2 as lherzolite Bri3. Gel +6 matches well the target composition (that is, the glass in partial melting experiment MBK+6), except that it is ≈ 10 % richer in Na2O. -

Lunar Crater Volcanic Field (Reveille and Pancake Ranges, Basin and Range Province, Nevada, USA)
Research Paper GEOSPHERE Lunar Crater volcanic field (Reveille and Pancake Ranges, Basin and Range Province, Nevada, USA) 1 2,3 4 5 4 5 1 GEOSPHERE; v. 13, no. 2 Greg A. Valentine , Joaquín A. Cortés , Elisabeth Widom , Eugene I. Smith , Christine Rasoazanamparany , Racheal Johnsen , Jason P. Briner , Andrew G. Harp1, and Brent Turrin6 doi:10.1130/GES01428.1 1Department of Geology, 126 Cooke Hall, University at Buffalo, Buffalo, New York 14260, USA 2School of Geosciences, The Grant Institute, The Kings Buildings, James Hutton Road, University of Edinburgh, Edinburgh, EH 3FE, UK 3School of Civil Engineering and Geosciences, Newcastle University, Newcastle, NE1 7RU, UK 31 figures; 3 tables; 3 supplemental files 4Department of Geology and Environmental Earth Science, Shideler Hall, Miami University, Oxford, Ohio 45056, USA 5Department of Geoscience, 4505 S. Maryland Parkway, University of Nevada Las Vegas, Las Vegas, Nevada 89154, USA CORRESPONDENCE: gav4@ buffalo .edu 6Department of Earth and Planetary Sciences, 610 Taylor Road, Rutgers University, Piscataway, New Jersey 08854-8066, USA CITATION: Valentine, G.A., Cortés, J.A., Widom, ABSTRACT some of the erupted magmas. The LCVF exhibits clustering in the form of E., Smith, E.I., Rasoazanamparany, C., Johnsen, R., Briner, J.P., Harp, A.G., and Turrin, B., 2017, overlapping and colocated monogenetic volcanoes that were separated by Lunar Crater volcanic field (Reveille and Pancake The Lunar Crater volcanic field (LCVF) in central Nevada (USA) is domi variable amounts of time to as much as several hundred thousand years, but Ranges, Basin and Range Province, Nevada, USA): nated by monogenetic mafic volcanoes spanning the late Miocene to Pleisto without sustained crustal reservoirs between the episodes.