Environmental Issues of Fish Farming in Offshore Waters: Perspectives, Concerns and Research Needs
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

SUSTAINABLE FISHERIES and RESPONSIBLE AQUACULTURE: a Guide for USAID Staff and Partners
SUSTAINABLE FISHERIES AND RESPONSIBLE AQUACULTURE: A Guide for USAID Staff and Partners June 2013 ABOUT THIS GUIDE GOAL This guide provides basic information on how to design programs to reform capture fisheries (also referred to as “wild” fisheries) and aquaculture sectors to ensure sound and effective development, environmental sustainability, economic profitability, and social responsibility. To achieve these objectives, this document focuses on ways to reduce the threats to biodiversity and ecosystem productivity through improved governance and more integrated planning and management practices. In the face of food insecurity, global climate change, and increasing population pressures, it is imperative that development programs help to maintain ecosystem resilience and the multiple goods and services that ecosystems provide. Conserving biodiversity and ecosystem functions are central to maintaining ecosystem integrity, health, and productivity. The intent of the guide is not to suggest that fisheries and aquaculture are interchangeable: these sectors are unique although linked. The world cannot afford to neglect global fisheries and expect aquaculture to fill that void. Global food security will not be achievable without reversing the decline of fisheries, restoring fisheries productivity, and moving towards more environmentally friendly and responsible aquaculture. There is a need for reform in both fisheries and aquaculture to reduce their environmental and social impacts. USAID’s experience has shown that well-designed programs can reform capture fisheries management, reducing threats to biodiversity while leading to increased productivity, incomes, and livelihoods. Agency programs have focused on an ecosystem-based approach to management in conjunction with improved governance, secure tenure and access to resources, and the application of modern management practices. -

FAO Fisheries & Aquaculture
Food and Agriculture Organization of the United Nations Fisheries and for a world without hunger Aquaculture Department Fishing Techniques Midwater Pair Trawling Main Components Aquatic species Target Species Semi-pelagic/demersal species Atlantic herring European pilchard(=Sardine) Seabream Hake Seabass European sprat Target Species Pelagic species Gear types: Midwater pair trawls Midwater pair trawls It has similar characteristics as midwater trawls used with otter boards. Vessel types: Pair trawlers In the wet-fish trawler the fish is kept in the hold in the fresh/"wet" condition. Characteristics Midwater pair trawling Species Environment Midwater pair trawling can be effective in different situations: when fish are aggregated into large dense shoals and when (at another season or time of the day or according to physiological status) fishes are regularly distributed within a given water layer. In addition to the difference it makes whether the FAO Fisheries and Aquaculture Department fish are aggregated in a small volume or spread within a large one, fish may swim (and avoid the net) at different speeds according to its own physiological status and/or other external conditions. As a result, in addition to the fish which is targeted, other different conditions will affect the design and size of the midwater trawl, as well as the towing speed. Fishing Gear A midwater pair trawl has roughly similar design as other midwater trawls. Midwater pair trawls might, however, be designed to have a more rectangular opening than ordinary midwater otter trawls. Midwater pair trawls might be rigged with two towing warps from each vessel or alternatively with one towing warp from each vessel and a bridle arrangement. -

Cobia Database Articles Final Revision 2.0, 2-1-2017
Revision 2.0 (2/1/2017) University of Miami Article TITLE DESCRIPTION AUTHORS SOURCE YEAR TOPICS Number Habitat 1 Gasterosteus canadus Linné [Latin] [No Abstract Available - First known description of cobia morphology in Carolina habitat by D. Garden.] Linnaeus, C. Systema Naturæ, ed. 12, vol. 1, 491 1766 Wild (Atlantic/Pacific) Ichthyologie, vol. 10, Iconibus ex 2 Scomber niger Bloch [No Abstract Available - Description and alternative nomenclature of cobia.] Bloch, M. E. 1793 Wild (Atlantic/Pacific) illustratum. Berlin. p . 48 The Fisheries and Fishery Industries of the Under this head was to be carried on the study of the useful aquatic animals and plants of the country, as well as of seals, whales, tmtles, fishes, lobsters, crabs, oysters, clams, etc., sponges, and marine plants aml inorganic products of U.S. Commission on Fisheries, Washington, 3 United States. Section 1: Natural history of Goode, G.B. 1884 Wild (Atlantic/Pacific) the sea with reference to (A) geographical distribution, (B) size, (C) abundance, (D) migrations and movements, (E) food and rate of growth, (F) mode of reproduction, (G) economic value and uses. D.C., 895 p. useful aquatic animals Notes on the occurrence of a young crab- Proceedings of the U.S. National Museum 4 eater (Elecate canada), from the lower [No Abstract Available - A description of cobia in the lower Hudson Eiver.] Fisher, A.K. 1891 Wild (Atlantic/Pacific) 13, 195 Hudson Valley, New York The nomenclature of Rachicentron or Proceedings of the U.S. National Museum Habitat 5 Elacate, a genus of acanthopterygian The universally accepted name Elucate must unfortunately be supplanted by one entirely unknown to fame, overlooked by all naturalists, and found in no nomenclator. -

Integrated Multi-Trophic Aquaculture Systems: a Solution for Sustainability
Integrated multi-trophic aquaculture systems: A solution for sustainability Kapil S. Sukhdhane1, V. Kripa2, Divu, D.1, Vinay Kumar Vase1 and Suresh Kumar Mojjada1 1. Veraval Regional Center of ICAR - Central Marine Fisheries Research Institute, Bhidia plot, Veraval, Gujarat 362269, India; 2. ICAR-Central Marine Fisheries Research Institute, Ernakulam North, P.O., Cochin 682018, India. Marine aquaculture is increasingly seen as an alternative organic and inorganic) when cultivated alongside fed fi sh to fi shing to provide a growing human population with species (Chopin et al. 2001; Neori et al. 2004; Troell et al. high-quality protein. Capture fi sheries output is falling short of 2003). world demand, and annual consumption of seafood has been rising and doubled over the last three decades (FAO, 2000). Fed aquaculture species (e.g. fi nfi sh/shrimps) are combined, Aquaculture production has surpassed supplies from capture in the appropriate proportions, with organic extractive fi sheries and contributed around 51% to global fi sh production aquaculture species (e.g. suspension feeders/deposit in 2014. Over the past three decades aquaculture production feeders/herbivorous fi sh) and inorganic extractive aquaculture increased from 6.2 million tonnes in 1983 to 73.8 million species (e.g. seaweeds), for a balanced ecosystem manage- tonnes in 2014 (FAO, 2016). This achievement was possible ment approach that takes into consideration site specifi city, mainly because of the commercialisation of farm produced operational limits, and food safety guidelines and regulations. aquatic animals such as shrimp, salmon, bivalves, tilapia The integrated in IMTA refers to the more intensive cultivation and catfi sh. -

Offshore Aquaculture in the United States: Economic Considerations, Implications & Opportunities
Offshore Aquaculture in the United States: Economic Considerations, Implications & Opportunities July 2008 U.S. Department of Commerce National Oceanic & Atmospheric Administration Silver Spring, Maryland NOAA Technical Memorandum NMFS F/SPO-103 You may download an electronic version of this report from: http://aquaculture.noaa.gov This document should be cited as follows: Rubino, Michael (editor). 2008. Offshore Aquaculture in the United States: Economic Considerations, Implications & Opportunities. U.S. Department of Commerce; Silver Spring, MD; USA. NOAA Technical Memorandum NMFS F/SPO-103. 263 pages. For more information: NOAA Aquaculture Program 1315 East-West Hwy. SSMC #3 – Room 13117 Silver Spring MD 20910 (301) 713-9079 E-mail: [email protected] Website: http://aquaculture.noaa.gov Offshore Aquaculture in the United States: Economic Considerations, Implications & Opportunities Prepared by the NOAA Aquaculture Program From technical contributions by James L. Anderson, John Forster, Di Jin, James E. Kirkley, Gunnar Knapp, Colin E. Nash, Michael Rubino, Gina L. Shamshak, Diego Valderrama NOAA Aquaculture Program 1315 East-West Hwy. SSMC #3 – Room 13117 Silver Spring MD 20910 July 2008 U.S. DEPARTMENT OF COMMERCE Carlos M. Gutierrez, Secretary NATIONAL OCEANIC & ATMOSPHERIC ADMINISTRATION Vice Admiral Conrad C. Lautenbacher, Jr. USN (Ret.), Administrator NATIONAL MARINE FISHERIES SERVICE James Balsiger, Assistant Administrator for Fisheries This page intentionally left blank. TABLE OF CONTENTS Chapter 1: Introduction …………………………………………………………….. 1 Michael Rubino Chapter 2: Economic Potential for U.S. Offshore Aquaculture: An Analytical Approach ………………………………………………. 15 Gunnar Knapp Chapter 3: Emerging Technologies in Marine Aquaculture ……………………….. 51 John Forster Chapter 4: Future Aquaculture Feeds and Feed Costs: The Role of Fish Meal and Fish Oil …………………………………… 73 Gina Shamshak & James Anderson Chapter 5: Lessons from the Development of the U.S. -

Gulf Council Aquaculture Faqs
Gulf of Mexico Fishery Management Council Aquaculture Fishery Management Plan Frequently Asked Questions What is offshore aquaculture? Offshore aquaculture is the rearing of aquatic organisms in controlled environments (e.g., cages or net pens) in federally managed areas of the ocean. Federally managed areas of the Gulf of Mexico begin where state jurisdiction ends and extend 200 miles offshore, to the outer limit of the U.S. Exclusive Economic Zone (EEZ). Why conduct aquaculture offshore? Offshore aquaculture is desirable for several reasons. First, there are fewer competing uses (e.g., fishing and recreation) farther from shore. Second, the deeper water makes it a desirable location with more stable water quality characteristics for rearing fish and shellfish. The stronger waterflows offshore also mitigate environmental effects such as nutrient and organic loading. Are there currently any offshore aquaculture operations in federal waters of the United States? Currently there are no commercial finfish offshore aquaculture operations in U.S. federal waters. There are currently 25 permit holders for live rock aquaculture in the EEZ. There are also several aquaculture operations conducting research and commercial production in state waters, off the coasts of California, New Hampshire, Hawaii, Washington, Maine, and Florida. Why did the Gulf of Mexico Fishery Management Council develop a Fishery Management Plan (FMP) for regulating offshore marine aquaculture in the Gulf of Mexico? The current Federal permitting process for offshore aquaculture is of limited duration and is not intended for the large-scale production of fish, making commercial aquaculture in federal waters impracticable at this time. Offshore aquaculture could help meet consumers’ growing demand for seafood with high quality local supply, create jobs in coastal communities, help maintain working waterfronts, and reduce the nation’s dependence on seafood imports. -

Aquaponics Division of Agriculture RESOURCES April 2015
Alaska Department of Fact Sheet NATURAL Aquaponics Division of Agriculture RESOURCES April 2015 What is Aquaponics? a manner that allows it to be certified, check with the organic Aquaponics is a food production system that links hydroponic certification agency prior to constructing the system. crop production with aquaculture (fish farming). Unlike open- water aquaculture, aquaponics generally operates on land, BE AWARE – Aquaponics is regulated in Alaska and results in production of a food crop. Aquaponics is not a To ensure that your aquaponics system is legal, check with new concept; crops and fish have been grown together for the Alaska Department of Fish & Game. Growing fish for many centuries. However, aquaponics systems are becoming human consumption (including by aquaponic methods) is very popular due to their efficiency, high productivity, and NOT legal in the State of Alaska, and the importation and minimal impact to the environment. transport of most live fish in the state is prohibited without a permit. Fish that are strictly ornamental (such as goldfish) and How Does Aquaponics Work? not raised for human consumption or sport fishing purposes Aquaponics takes advantage of the fact that plants can thrive in the nutrient-rich water from fish ponds. Plants and may be imported into the state and used in a closed system, associated microbes convert byproducts such as ammonia but they may not be reared in or released into the waters of and CO2 to beneficial products such as nitrate and oxygen, the state. Fish wastes and wastewater from ornamental fish in a semi-closed system. may also not be released to the waters of the state. -
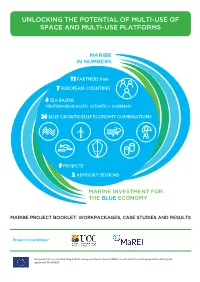
Maribe in Numbersin Numbers
UNLOCKING THE POTENTIAL OF MULTI-USE OF SPACE AND MULTI-USE PLATFORMS MARIBEMARIBE IN NUMBERSIN NUMBERS 11 PARTNERS from 7 EUROPEAN COUNTRIES 4 SEA BASINS MEDITERRANEAN, BALTIC, ALTANTIC & CARIBBEAN 24 BLUE GROWTH/BLUE ECONOMY COMBINATIONS 9 PROJECTS 2 ADVISORY SESSIONS MARINEMARINE INVESTMENT INVESTMENT FOR FORTHE BLUETHE BLUE ECONOMY ECONOMY MARIBE PROJECT BOOKLET: WORKPACKAGES, CASE STUDIES AND RESULTS Project coordinator This project has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No 652629 UNLOCKING THE POTENTIAL OF MULTI-USE UNLOCKING THE POTENTIAL OF MULTI-USE OF SPACE AND MULTI-USE PLATFORMS OF SPACE AND MULTI-USE PLATFORMS Contents Partner Organisations and List of Abbreviations ................................................................................ .2 Introduction ....................................................................................................................................... 3 WP Summaries ................................................................................................................................... 6 Work Package -‐ 4 Socio-‐Economic Trends and EU Policy in the Offshore Economy ..................... 6 WP 5 -‐ Te chnical and Non-‐technical Challenges, Regional and Sectoral......................... ............... 9 WP 6 -‐ In vestment community consultation and commitment....................................... ............. 10 WP 7 -‐ Business Model Mapping and Assessment...................................................................... -

Impact of Bottom Trawling on Sediment Biogeochemistry: a Modelling Approach Emil De Borger1,2, Justin Tiano2,1, Ulrike Braeckman1, Adriaan D
https://doi.org/10.5194/bg-2020-328 Preprint. Discussion started: 21 September 2020 c Author(s) 2020. CC BY 4.0 License. Impact of bottom trawling on sediment biogeochemistry: a modelling approach Emil De Borger1,2, Justin Tiano2,1, Ulrike Braeckman1, Adriaan D. Rijnsdorp3, Karline Soetaert2,1. 1Ghent University, Department of Biology, Marine Biology Research Group, Krijgslaan 281/S8, 9000 Ghent, Belgium 5 2Royal Netherlands Institute of Sea Research (NIOZ), Department of Estuarine and Delta Systems, and Utrecht University, Korringaweg 7, P.O. Box 140, 4401 NT Yerseke, The Netherlands 3Wageningen Marine Research, Wageningen University & Research, IJmuiden, The Netherlands Correspondence to : Emil De Borger ([email protected]) Abstract 10 Bottom trawling in shelf seas can occur more than 10 times per year for a given location. This affects the benthic metabolism, through a mortality of the macrofauna, resuspension of organic matter from the sediment, and alterations of the physical sediment structure. However, the trawling impacts on organic carbon mineralization and associated processes are not well known. Using a modelling approach, the effects of increasing trawling frequencies on early diagenesis were studied in five different sedimentary environments, simulating the effects of a deep penetrating gear (e.g. a tickler chain beam trawl) and a 15 shallower, more variable penetrating gear (e.g. an electric pulse trawl). Trawling events strongly increased oxygen and nitrate concentrations in surface sediment layers, and led to significantly lower amounts of ammonium (43 – 99 % reduction) and organic carbon in the top 10 cm of the sediment (62 – 96 % reduction). As a result, total mineralization rates in the sediment were decreased by up to 28 %. -

BOTTOM TRAWLING IMPACTS in the BALTIC Editorial and Production Team: Hannah Griffiths Berggren, Valerie De Liedekerke, Ottilia Thoreson, Evan Jeffries, Brandline
2020 BALTIC A SEA UNDER PRESSURE: BOTTOM TRAWLING IMPACTS IN THE BALTIC Editorial and Production Team: Hannah Griffiths Berggren, Valerie de Liedekerke, Ottilia Thoreson, Evan Jeffries, Brandline Authors: Valerie de Liedekerke, Ottilia Thoreson, Sian Owen, Hannah Griffiths Berggren Special thanks: Professor Rashid Sumaila (Institute for the Oceans and Fisheries – The University of British Columbia); Louise Lieberknecht, Georgios Fylakis, Màrk Aguera and Torben Dedring (GRID-Arendal); Andreas Struck (Navama – Technology for Nature); Marco Milardi (Principal Scientist, Fisheries New Zealand - Tini a Tangaroa, Ministry for Primary Industries - Manatū Ahu Matua). We would also like to thank everyone who kindly provided data and reviewed the report. This report was made possible thanks to a substantial contribution of funds from Monika Selkman. Cover Photo: © Stefan Rosengren / Alamy Stock Photo © Alamy Stock Photo / Solvin Zankl (montage) 2 • WWF BALTIC ECOREGION PROGRAMME 2020 CONTENTS i. EXECUTIVE SUMMARY 4 1. BACKGROUND 6 1a. The Baltic Sea under pressure 6 1b. What is ‘bottom trawling’? 8 2. THE IMPACTS OF BOTTOM TRAWLING 10 2a. Physical and biological impacts 10 2b. The future of the Baltic Sea fishing fleet 17 3. RELEVANT POLICY FRAMEWORKS AND COMMITMENTS 22 3a. Global policy frameworks 22 3b. European policy commitments 24 3c. Regional Baltic policy commitments 27 3d. Examples of how governance is failing 28 4. SHOWCASING POSITIVE MANAGEMENT EXAMPLES 30 5. RECOMMENDATIONS 38 ACRONYMS 40 REFERENCES 41 © Alamy Stock Photo / Solvin Zankl (montage) 3 • WWF BALTIC ECOREGION PROGRAMME 2020 EXECUTIVE SUMMARY The Baltic Sea is surrounded by nine countries, whose accordingly. Forty per cent of the entire Baltic seafloor, futures are tightly connected to each other and to their an area of 180,000km2, has been disturbed by maritime shared marine resources. -

FAO Fisheries & Aquaculture
Food and Agriculture Organization of the United Nations Fisheries and for a world without hunger Aquaculture Department Fishing Techniques Shrimp Otter Trawling Main Components Aquatic species Target Species Shrimps Gear types: Single boat bottom otter trawls Single boat bottom otter trawls A single boat bottom otter trawl is a cone-shaped net consisting of a body, normally made from two, four and sometimes more panels, closed by one or two codends and with lateral wings extending forward from the opening. A bottom trawl is kept open horizontally by two otter boards. A boat can be rigged to tow a single or two parallel trawls from the stern or from two outriggers. Vessel types: Otter trawlers These are trawlers on which the fish is preserved by freezing. Characteristics Shrimp otter trawling Species Environment Shrimps constitute one of the most valuable groups of marine species resources. Approximately 2 million tonnes are captured annually in world's fisheries. Targets in shrimp otter trawling encompasses a wide range of species in both tropical and temperate waters. Fishing Gear Trawls used in otter shrimp trawling encompass a range of designs and sizes. Shrimp trawls are typical made in relatively small meshes, with 20-60 mm in the codend. The mesh size in the belly part of the trawls seldom exceed 80 mm. Some larger shrimp trawls may have larger meshes in the wings, up till 100-200 mm. Vertical opening of shrimp trawls may range from less than 1 till 15-20 meters. Characteristic for otter shrimp trawling is that one or two trawls are towed from the stern of a vessel. -

Contribución Al Desarrollo Sostenible
Contribution to Sustainable Development 2019 - Nueva Pescanova Group FISHING – NAMIBIA – NOVANAM GENERAL SDG SDG SDG SDG SDG SDG SDG SDG SDG SDG SDG SDG SDG SDG SDG SDG SDG SPECIFIC PLAN CODE ACTION'S TITLE PLAN 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 Respecting the 12.2 - 14.2 NAM-PL-01 ISO 14001 certification 2.4 9.4 natural environment 12.6 14.7 Respecting the Private Standard of Sustainable 12.2 14.2 NAM-PL-02 2.4 9.4 natural environment Fishing of the Nueva Pescanova Group 12.6 14.7 Respecting the Seabird bycatch reduction programme 12.2 NAM-PL-03 9.4 14.7 natural environment (use of tori lines) 12.6 Respecting the Design of new seabed-friendly 12.2 14.2 NAM-PL-04 9.4 natural environment trawling nets 12.6 14.7 Reduction of water consumption - 12.2 Efficient consumption NAM-PL-05 6.4 9.4 Our glazing 12.6 Common PLANET Reduction of water consumption - 6.3 12.2 Efficient consumption NAM-PL-06 9.4 desalination 6.4 12.4 Circular valorisation organic of fish co- 9.2 12.5 Zero emissions NAM-PL-07 products as fish meal in Lüderitz 9.4 12.6 Circular valorisation of organic fish co- 9.2 12.5 Zero emissions NAM-PL-08 products as fish meal in Walvis Bay 9.4 12.6 Responsible waste management - 12.4 Zero emissions NAM-PL-09 9.4 11.6 management plan 12.5 12.2 13.2 14.2 Zero emissions NAM-PL-10 Improved design of trawl doors 9.4 12.a 13.a 14.7 II Report on the Contribution to Sustainable Development – CSR strategy of the Nueva Pescanova Group – FISHING – NAMIBIA– NOVANAM Page 1 of 7 Contribution to Sustainable Development 2019 - Nueva Pescanova