Tropical Deforestation and Species Extinction
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Land-Use, Land-Cover Changes and Biodiversity Loss - Helena Freitas
LAND USE, LAND COVER AND SOIL SCIENCES – Vol. I - Land-Use, Land-Cover Changes and Biodiversity Loss - Helena Freitas LAND-USE, LAND-COVER CHANGES AND BIODIVERSITY LOSS Helena Freitas University of Coimbra, Portugal Keywords: land use; habitat fragmentation; biodiversity loss Contents 1. Introduction 2. Primary Causes of Biodiversity Loss 2.1. Habitat Degradation and Destruction 2.2. Habitat Fragmentation 2.3. Global Climate Change 3. Strategies for Biodiversity Conservation 3.1. General 3.2. The European Biodiversity Conservation Strategy 4. Conclusions Glossary Bibliography Biographical Sketch Summary During Earth's history, species extinction has probably been caused by modifications of the physical environment after impacts such as meteorites or volcanic activity. On the contrary, the actual extinction of species is mainly a result of human activities, namely any form of land use that causes the conversion of vast areas to settlement, agriculture, and forestry, resulting in habitat destruction, degradation, and fragmentation, which are among the most important causes of species decline and extinction. The loss of biodiversity is unique among the major anthropogenic changes because it is irreversible. The importance of preserving biodiversity has increased in recent times. The global recognition of the alarming loss of biodiversity and the acceptance of its value resultedUNESCO in the Convention on Biologi – calEOLSS Diversity. In addition, in Europe, the challenge is also the implementation of the European strategy for biodiversity conservation and agricultural policies, though it is increasingly recognized that the strategy is limitedSAMPLE by a lack of basic ecological CHAPTERS information and indicators available to decision makers and end users. We have reached a point where we can save biodiversity only by saving the biosphere. -

Desertification and Deforestation in Africa - R
LAND USE, LAND COVER AND SOIL SCIENCES – Vol. V – Desertification and Deforestation in Africa - R. Penny DESERTIFICATION AND DEFORESTATION IN AFRICA R. Penny Environmental and Developmental Consultant/Practitioner, Cape Town, South Africa Keywords: arid, semi-arid, dry sub-humid, drought, drylands, land degradation, land tenure, sustainability Contents 1. Introduction 2. Global Context 3. Land Degradation in Africa Today 3.1. Geographical Regions 3.2. Socio-Economic Aspects 4. Causes and Consequences 4.1. Drought and Other Disasters 4.2. Water Quality and Availability 4.3. Loss of Vegetative Cover 4.4. Loss of Soil Fertility 4.5. Poverty and Population 4.6. Effect of Land Tenure 4.7. Health 5. Combating Desertification 5.1. Past Trends 5.2. Current Attempts to Combat Desertification 5.3. Synergy of the Three Sustainable Development Conventions 5.4. The Role of Science and Technology in Combating Desertification 5.5. Synergy in Environmental Policy Development 6. Future Perspectives: The Way Forward 7. Conclusions Glossary Bibliography Biographical Sketch Summary UNESCO – EOLSS Africa is particularly vulnerable to desertification. Two thirds of the continent consists of desert or drylands.SAMPLE The obvious causes of desertificatiCHAPTERSon and deforestation consist of major ecosystem changes, such as land conversion for various purposes, over- dependence on natural resources and several forms of unsustainable land use. However, the issue of desertification is inseparable from social problems such as poverty and land tenure issues. Politics, war and national disasters affect the movements of people and thus impact on the land. International trade policies as well play a part in land management and/or exploitation. -

Energy Development's Impacts on the Wildlife, Landscapes, And
Losing Ground: Energy Development’s Impacts on the Wildlife, Landscapes, and Hunting Traditions of the American West A Report by the National Wildlife Federation and the Natural Resources Defense Council Barbara Wheeler he iconic game species of the American wildlife are an ecological marker for the health of non- West are in perilous decline, as migratory game species, which are also becoming increasingly T animals lose ground to energy development vulnerable to the effects of energy development. and habitat destruction in southeast Montana and northeast Wyoming. Sage-grouse, mule deer, and Bountiful wildlife populations are a major part of the pronghorn are facing decreasing herd sizes and cultures and economies of Montana and Wyoming. downward long-term population trends, which Deteriorations in habitat quality associated with threaten the continued viability of these species in energy development can negatively affect wildlife the coming decades. Of the species considered, only populations and, in turn, impact hunters, wildlife- elk populations are forecast to rebound and stabilize watchers, and the tourism industry as a whole, which brings millions of people and billions of dollars to that additional habitat loss or degradation from the region every year. These impacts justify the need from historic lows, though there is significant concern energy development could stall population growth and for land management reforms and for wide-scale further displace the species. Simultaneously, big game investments in game and non-game wildlife protection Losing Ground Jack Dempsey at state and federal levels to offset the increasing impact on wildlife from energy development. Oregon Department of Fish and Wildlife A study commissioned by the National Wildlife Federation and the Natural Resources Defense A study commissioned by the Council analyzes trends in population and hunting National Wildlife Federation and opportunities for mule deer, pronghorn, elk, and the Natural Resources Defense greater sage-grouse. -

Road Impact on Deforestation and Jaguar Habitat Loss in The
ROAD IMPACT ON DEFORESTATION AND JAGUAR HABITAT LOSS IN THE MAYAN FOREST by Dalia Amor Conde Ovando University Program in Ecology Duke University Date:_______________________ Approved: ___________________________ Norman L. Christensen, Supervisor ___________________________ Alexander Pfaff ___________________________ Dean L. Urban ___________________________ Randall A. Kramer Dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy in the University Program in Ecology in the Graduate School of Duke University 2008 ABSTRACT ROAD IMPACT ON DEFORESTATION AND JAGUAR HABITAT LOSS IN THE MAYAN FOREST by Dalia Amor Conde Ovando University Program in Ecology Duke University Date:_______________________ Approved: ___________________________ Norman L. Christensen, Supervisor ___________________________ Alexander Pfaff ___________________________ Dean L. Urban ___________________________ Randall A. Kramer An abstract of a dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy in the University Program in Ecology in the Graduate School of Duke University 2008 Copyright by Dalia Amor Conde Ovando 2008 Abstract The construction of roads, either as an economic tool or as necessity for the implementation of other infrastructure projects is increasing in the tropical forest worldwide. However, roads are one of the main deforestation drivers in the tropics. In this study we analyzed the impact of road investments on both deforestation and jaguar habitat loss, in the Mayan Forest. As well we used these results to forecast the impact of two road investments planned in the region. Our results show that roads are the single deforestation driver in low developed areas, whether many other drivers play and important role in high developed areas. In the short term, the impact of a road in a low developed area is lower than in a road in a high developed area, which could be the result of the lag effect between road construction and forest colonization. -

Hidden Deforestation in the Brazil - China Beef and Leather Trade 1
Hidden deforestation in the Brazil - China beef and leather trade 1 Hidden deforestation in the Brazil - China beef and leather trade Christina MacFarquhar, Alex Morrice, Andre Vasconcelos August 2019 Key points: China is Brazil’s biggest export market for cattle products, • Cattle ranching is the leading direct driver of deforestation which are a major driver of deforestation and other native and other native vegetation clearance in Brazil, and some vegetation loss in Brazil. This brief identifies 43 companies international beef and leather supply chains are linked to worldwide that are highly exposed to deforestation risk through these impacts. the Brazil-China beef and leather trade, and which have significant potential to help reduce this risk. The brief shows • China (including Hong Kong) is Brazil’s biggest importer of which of these companies have published policies to address beef and leather, and many companies linked to this trade are deforestation risk related to these commodities. It also reveals exposed to deforestation risk. the supplier-buyer relationships between these companies, • We identify 43 companies globally that are particularly exposed and how their connections may mean even those buyers with to the deforestation risk associated with the Brazil-China beef commitments to reduce or end deforestation may not be able to and leather trade and have the potential to reduce these risks. meet them. It then makes recommendations for the next steps companies can take to address deforestation risk. • Most of these companies have not yet published sustainable sourcing policies to address this risk. The companies include cattle processors operating in Brazil, processors and manufacturers operating in China, and • Most appear unable to guarantee that their supply chains are manufacturers and retailers headquartered in Europe and the deforestation-free, because they, or a supplier, lack a strong United States of America (US). -

Desertification and Agriculture
BRIEFING Desertification and agriculture SUMMARY Desertification is a land degradation process that occurs in drylands. It affects the land's capacity to supply ecosystem services, such as producing food or hosting biodiversity, to mention the most well-known ones. Its drivers are related to both human activity and the climate, and depend on the specific context. More than 1 billion people in some 100 countries face some level of risk related to the effects of desertification. Climate change can further increase the risk of desertification for those regions of the world that may change into drylands for climatic reasons. Desertification is reversible, but that requires proper indicators to send out alerts about the potential risk of desertification while there is still time and scope for remedial action. However, issues related to the availability and comparability of data across various regions of the world pose big challenges when it comes to measuring and monitoring desertification processes. The United Nations Convention to Combat Desertification and the UN sustainable development goals provide a global framework for assessing desertification. The 2018 World Atlas of Desertification introduced the concept of 'convergence of evidence' to identify areas where multiple pressures cause land change processes relevant to land degradation, of which desertification is a striking example. Desertification involves many environmental and socio-economic aspects. It has many causes and triggers many consequences. A major cause is unsustainable agriculture, a major consequence is the threat to food production. To fully comprehend this two-way relationship requires to understand how agriculture affects land quality, what risks land degradation poses for agricultural production and to what extent a change in agricultural practices can reverse the trend. -

Human Population Growth and Its Implications on the Use and Trends of Land Resources in Migori County, Kenya
HUMAN POPULATION GROWTH AND ITS IMPLICATIONS ON THE USE AND TRENDS OF LAND RESOURCES IN MIGORI COUNTY, KENYA PAULINE TOLO OGOLA A Thesis Submitted in Partial Fulfilment of the Requirements for the Award of the Degree of Master of Environmental Studies (Agroforestry and Rural Development) in the School of Environmental Studies of Kenyatta University NOVEMBR, 2018 1 DEDICATION To my loving parents, Mr. and Mrs. Ogola, With long life He will satisfy you i ACKNOWLEDGEMENT First of all, I am grateful to the Man above who gave me strength and health throughout this study. For sure, His goodness and Mercies are new every day. Secondly, I am greatly indebted to my supervisors Dr. Letema and Dr. Obade for their wise counsel and patience. Thirdly, I would like to convey my utmost gratitude to my parents and siblings for their moral support and prayers. Special thanks to my brother Stephen Ogeda for supporting me financially. Finally, I wish to express many thanks to my colleagues at the Regional Centre for Mapping of Resources for Development and friends who have offered their support in kind and deed. ii TABLE OF CONTENTS DECLARATION………………………………………………………………………… Error! Bookmark not defined. DEDICATION…………………………………………………………………………...i ACKNOWLEDGEMENT……………………………………………………………...ii LIST OF TABLES……………………………………………………………………...vi LIST OF FIGURES……………………………………………………………………vii ABBREVIATIONS AND ACRONYMS……………………………………………….viii ABSTRACT………………………………………………………………………………i x CHAPTER ONE: INTRODUCTION…………………………………………………..1 1.1 Background to the Problem ......................................................................................... -
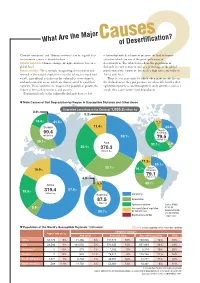
What Are the Major Causes of Desertification?
What Are the Major Causesof Desertification? ‘Climatic variations’ and ‘Human activities’ can be regarded as relationship with development pressure on land by human the two main causes of desertification. activities which are one of the principal causes of Climatic variations: Climate change, drought, moisture loss on a desertification. The table below shows the population in global level drylands by each continent and as a percentage of the global Human activities: These include overgrazing, deforestation and population of the continent. It reveals a high ratio especially in removal of the natural vegetation cover(by taking too much fuel Africa and Asia. wood), agricultural activities in the vulnerable ecosystems of There is a vicious circle by which when many people live in arid and semi-arid areas, which are thus strained beyond their the dryland areas, they put pressure on vulnerable land by their capacity. These activities are triggered by population growth, the agricultural practices and through their daily activities, and as a impact of the market economy, and poverty. result, they cause further land degradation. Population levels of the vulnerable drylands have a close 2 ▼ Main Causes of Soil Degradation by Region in Susceptible Drylands and Other Areas Degraded Land Area in the Dryland: 1,035.2 million ha 0.9% 0.3% 18.4% 41.5% 7.7 % Europe 11.4% 34.8% North 99.4 America million ha 32.1% 79.5 million ha 39.1% Asia 52.1% 5.4 26.1% 370.3 % million ha 11.5% 33.1% 30.1% South 16.9% 14.7% America 79.1 million ha 4.8% 5.5 40.7% Africa -

Nomadic-Colonial Life Strategies Enable Paradoxical Survival and Growth Despite Habitat Destruction Zhi Xuan Tan1, Kang Hao Cheong2*
RESEARCH ARTICLE Nomadic-colonial life strategies enable paradoxical survival and growth despite habitat destruction Zhi Xuan Tan1, Kang Hao Cheong2* 1Yale University, New Haven, United States; 2Engineering Cluster, Singapore Institute of Technology, Singapore, Singapore Abstract Organisms often exhibit behavioral or phenotypic diversity to improve population fitness in the face of environmental variability. When each behavior or phenotype is individually maladaptive, alternating between these losing strategies can counter-intuitively result in population persistence–an outcome similar to the Parrondo’s paradox. Instead of the capital or history dependence that characterize traditional Parrondo games, most ecological models which exhibit such paradoxical behavior depend on the presence of exogenous environmental variation. Here we present a population model that exhibits Parrondo’s paradox through capital and history- dependent dynamics. Two sub-populations comprise our model: nomads, who live independently without competition or cooperation, and colonists, who engage in competition, cooperation, and long-term habitat destruction. Nomads and colonists may alternate behaviors in response to changes in the colonial habitat. Even when nomadism and colonialism individually lead to extinction, switching between these strategies at the appropriate moments can paradoxically enable both population persistence and long-term growth. Introduction *For correspondence: kanghao.cheong@singaporetech. Behavioral adaptation and phenotypic diversity are evolutionary meta-strategies that can improve edu.sg a population’s fitness in the presence of environmental variability. When behaviors or phenotypes are sufficiently distinct, a population can be understood as consisting of multiple sub-populations, Competing interests: The each following its own strategy. Counter-intuitively, even when each sub-population follows a los- authors declare that no ing strategy that will cause it to go extinct in the long-run, alternating or reallocating organisms competing interests exist. -

National Investment Plan to Reduce Deforestation and Forest Degradation (2018-2022)
Republic of Zambia Ministry of Lands and Natural Resources National Investment Plan to Reduce Deforestation and Forest Degradation (2018-2022) November 2017 -i- Executive Summary Zambia has approximately 49.9 million hectares of forest, representing approximately 66 percent of its total land mass and is subsequently one of the most forested countries in southern Africa. However, deforestation is a major problem, with annual rates estimated at around 250,000 to 300,000 hectares. Such numbers amount to the potential loss of 10 million hectares of forest in the next 30 years (IDLO, 2014). The key drivers of deforestation and forest degradation in Zambia are agricultural expansion (commercial and subsistence), heavy reliance on wood fuel – energy demand (charcoal and firewood), unsustainable timber extraction (both legal and illegal) and infrastructure development (e.g., mining and other large infrastructural developments). A study by UN-REDD, (2015) on the economic value of Zambia’s forest ecosystems showed that the direct and indirect values of forests are estimated to make a direct contribution equivalent to about 4.7% of gross domestic product (GDP) or US$957.5 million (using 2010 figures). However, when the multiplier effects of forestry and tourism-related activities on other sectors are considered, the overall or economy-wide contribution of forests to GDP is estimated to be at least 6.3% or US$1,277 million (Table 1). Forests are estimated to provide at least 1.4 million jobs, supporting 60% of rural Zambian households, heavily dependent upon the use of natural resources to supplement or sustain their livelihoods (UN-REDD., 2015). -

Habitat Evaluation: Guidance for the Review of Environmental Impact Assessment Documents
HABITAT EVALUATION: GUIDANCE FOR THE REVIEW OF ENVIRONMENTAL IMPACT ASSESSMENT DOCUMENTS EPA Contract No. 68-C0-0070 work Assignments B-21, 1-12 January 1993 Submitted to: Jim Serfis U.S. Environmental Protection Agency Office of Federal Activities 401 M Street, SW Washington, DC 20460 Submitted by: Mark Southerland Dynamac Corporation The Dynamac Building 2275 Research Boulevard Rockville, MD 20850 CONTENTS Page INTRODUCTION ... ...... .... ... ................................................. 1 Habitat Conservation .......................................... 2 Habitat Evaluation Methodology ................................... 2 Habitats of Concern ........................................... 3 Definition of Habitat ..................................... 4 General Habitat Types .................................... 5 Values and Services of Habitats ................................... 5 Species Values ......................................... 5 Biological diversity ...................................... 6 Ecosystem Services.. .................................... 7 Activities Impacting Habitats ..................................... 8 Land Conversion ....................................... 9 Land Conversion to Industrial and Residential Uses ............. 9 Land Conversion to Agricultural Uses ...................... 10 Land Conversion to Transportation Uses .................... 10 Timber Harvesting ...................................... 11 Grazing ............................................. 12 Mining ............................................. -

Deforestation and Social Risks in the Uk's Commodity
1 RISKY BUSINESS WORKING TOGETHER TO UNDERSTAND RISKS TO NATURAL CAPITAL DEFORESTATION AND SOCIAL RISKS IN THE UK’S COMMODITY SUPPLY CHAINS Lead authors: Steve Jennings, Richard Sheane and Catherine McCosker This report sets out research commissioned by WWF and the RSPB, carried out by consultancy 3Keel. It calculates the volume and source of UK imports (from 2011-15) of seven key forest-risk commodities: beef and leather, cocoa, palm oil, pulp and paper, rubber, soy, and timber. This data is then used to estimate the location and scale of the land footprint created by UK consumption of these commodities, and explores the risks associated with this footprint. The research uses publicly available data and sets out the assumptions made in estimating the footprint and risks. The findings of this research are summarised and analysed in a separate report, which also includes recommendations on what UK government, companies and citizens can do to address the risks. Risky Business: Understanding the UK’s overseas footprint for deforestation-risk commodities Available at: wwf.org.uk/riskybusiness Reports published October 2017. Contents 1 Executive summary ........................................................................................... 2 2 Introduction ........................................................................................................ 5 2.1 About this document ............................................................................................ 6 3 Overview of method .........................................................................................