David B. Morris Kennecott Corporation
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Federal Register/Vol. 79, No. 206/Friday, October 24, 2014/Rules
63540 Federal Register / Vol. 79, No. 206 / Friday, October 24, 2014 / Rules and Regulations ENVIRONMENTAL PROTECTION deletion of these parcels does not were to contain and control sources of AGENCY preclude future actions under contamination. Surface water and Superfund. ground water quality were not 40 CFR Part 300 DATES: This action is effective October specifically addressed in the remedies [EPA–HQ–SFUND–1983–0002; FRL–9918– 24, 2014. for these operable units. Site-wide water quality is specifically addressed in 37–Region 8] ADDRESSES: EPA has established a docket for this action under Docket OU12, which is an active operable unit. National Oil and Hazardous Identification No. EPA–HQ–SFUND– Under OU12, response action can be Substances Pollution Contingency 1983–0002. All documents in the docket conducted anywhere on the Site if Plan; National Priorities List: Partial are listed on the http:// needed to address releases that impact Deletion of the California Gulch www.regulations.gov Web site. Although or may impact water quality goals in the Superfund Site listed in the index, some information is Arkansas River. In OU4, OU5 and OU7, all responses actions have been AGENCY: Environmental Protection not publicly available, i.e., Confidential completed and institutional controls are Agency. Business Information or other in place. A responsiveness summary ACTION: Final rule. information whose disclosure is restricted by statute. Certain other was prepared and placed in both the SUMMARY: The Environmental Protection material, such as copyrighted material, docket, EPA–HQ–SFUND–1983–0002, Agency (EPA) Region 8 announces the is not placed on the Internet and will be on www.regulations.gov, and in the deletion of the Operable Unit 4 (OU4), publicly available only in hard copy local repository listed above. -

Principles of Extractive Metallurgy Lectures Note
PRINCIPLES OF EXTRACTIVE METALLURGY B.TECH, 3RD SEMESTER LECTURES NOTE BY SAGAR NAYAK DR. KALI CHARAN SABAT DEPARTMENT OF METALLURGICAL AND MATERIALS ENGINEERING PARALA MAHARAJA ENGINEERING COLLEGE, BERHAMPUR DISCLAIMER This document does not claim any originality and cannot be used as a substitute for prescribed textbooks. The information presented here is merely a collection by the author for their respective teaching assignments as an additional tool for the teaching-learning process. Various sources as mentioned at the reference of the document as well as freely available material from internet were consulted for preparing this document. The ownership of the information lies with the respective author or institutions. Further, this document is not intended to be used for commercial purpose and the faculty is not accountable for any issues, legal or otherwise, arising out of use of this document. The committee faculty members make no representations or warranties with respect to the accuracy or completeness of the contents of this document and specifically disclaim any implied warranties of merchantability or fitness for a particular purpose. BPUT SYLLABUS PRINCIPLES OF EXTRACTIVE METALLURGY (3-1-0) MODULE I (14 HOURS) Unit processes in Pyro metallurgy: Calcination and roasting, sintering, smelting, converting, reduction, smelting-reduction, Metallothermic and hydrogen reduction; distillation and other physical and chemical refining methods: Fire refining, Zone refining, Liquation and Cupellation. Small problems related to pyro metallurgy. MODULE II (14 HOURS) Unit processes in Hydrometallurgy: Leaching practice: In situ leaching, Dump and heap leaching, Percolation leaching, Agitation leaching, Purification of leach liquor, Kinetics of Leaching; Bio- leaching: Recovery of metals from Leach liquor by Solvent Extraction, Ion exchange , Precipitation and Cementation process. -

MOLYBDENUM by Michael J
MOLYBDENUM By Michael J. Magyar Domestic survey data and tables were prepared by Cindy C. Chen, statistical assistant, and the world production table was prepared by Linder Roberts, international data coordinator. Molybdenum is a refractory metallic element used principally until after the nearby Henderson deposit in Empire, CO, about as an alloying agent in cast iron, steel, and superalloys to 100 kilometers east, is exhausted. The Tonopah Mine in Nevada enhance hardenability, strength, toughness, and wear- and was being permanently closed. Molybdenum was produced as corrosion- resistance. To achieve desired metallurgical properties, a byproduct of copper production at the Bagdad and Sierrita molybdenum, primarily in the form of molybdic oxide (MoX) or Mines in Arizona and at the Bingham Canyon Mine in Utah. ferromolybdenum (FeMo), is frequently used in combination with The byproduct molybdenum recovery circuit at the Chino Mine or added to chromium, columbium (niobium), manganese, nickel, in New Mexico remained on care and maintenance. Montana tungsten, or other alloy metals. The versatility of molybdenum in Resources’ Continental Pit in Montana resumed operation enhancing a variety of alloy properties has ensured it a significant in November 2003, with the first shipments of molybdenite role in contemporary industrial technology, which increasingly concentrate expected in early 2004 (Platts Metals Week, 2003d). requires materials that are serviceable under high stress, expanded With byproduct molybdenum recovery at a copper mine, temperature ranges, and highly corrosive environments. Moreover, all mining costs associated with producing the molybdenum molybdenum finds significant use as a refractory metal in numerous concentrate are allocated to the primary metal (copper). -

Kennecott Utah Copper-Sustainable Over Time PHOTOS/VIDEO AUDIO
1 UNIVERSITY OF UTAH DEPARTMENT OF MINING ENGINEERING PRESENTATION to IMOA by Louie Cononelos “Kennecott Utah Copper-Sustainable Over Time PHOTOS/VIDEO AUDIO This is a story that had its beginning over 150 ago…and that story is still being written today. It began in Bingham Canyon, Utah located about 26 miles southwest of Salt Lake City, which was destined to become one of the greatest “mining camps” anywhere in the country. Mining in Utah, which was part of Spanish Mexico, can be traced back to Spanish miners in the mid-1700s. The start of mining in Utah, however, is credited to the United States Army in 1863. Troops under the command of Colonel Patrick Connor are credited with the discovery of Utah’s first mining claim and helping to form the first mining company and mining district in Bingham Canyon. The early mining at Bingham was underground with the exception of placer mining. 2 Bingham Canyon was a beehive of mining activity at the turn of the Century. Dozens of small companies dug tunnels and sank shafts in the mountains where they were mining lead, silver and gold ores…but not the low- grade copper ores that were in abundance and considered a nuisance. Then, along came Daniel C. Jackling, a 29-year-old metallurgical engineer, who with his partner, a mining engineer named Robert Gemmell, studied and assayed ore samples from the operations that dotted the canyon. They determined that there were vast tonnages of low- grade copper ore in the main mountain that divided the canyon…it was the kind of ore the mining companies tried to avoid because it interfered with the recovery of the metals they were mining. -

ENVIRONMENTAL CODE of PRACTICE Base Metals Smelters and Refineries
ENVIRONMENTAL CODE OF PRACTICE C ANADIAN E NVIRONMENTAL P ROTECTION A CT , 1999 First Edition Base Metals Smelters and Refineries March 2006 EPS 1/MM/11 E Metals Section Natural Resource Sectors Pollution Prevention Directorate Environmental Stewardship Branch Environment Canada Library and Archives Canada Cataloguing in Publication Main entry under title: Environmental Code of Practice for Base Metals Smelters and Refineries: Code of Practice, Canadian Environmental Protection Act, 1999. Issued also in French under title: Code de pratiques écologiques pour les fonderies et affineries de métaux communs : Code de pratique de la Loi canadienne sur la protection de l’environnement (1999). “First Edition”. Available also on the Internet. Includes bibliographical references. ISBN 0-662-42221-X Cat. no.: En84-34/2005E EPS 1/MM/11 E 1. Non-ferrous metal industries – Waste disposal – Canada. 2. Non-ferrous metals – Metallurgy – Environmental aspects – Canada. 3. Non-ferrous metals – Refining – Environmental aspects – Canada. 4. Smelting – Environmental aspects – Canada. 5. Best management practices (Pollution prevention) – Canada. i. Canada. Pollution Prevention Directorate. Metals Section. ii. Canada. Environment Canada. TD195.F6E58 2005 669'.028'6 C2005-980316-9 READERS’ COMMENTS Inquiries and comments on this Code of Practice, as well as requests for additional copies of the Code, should be directed to: Metals Section Natural Resources Sectors Division Pollution Prevention Directorate Environmental Stewardship Branch Environment Canada Place Vincent Massey 351 St. Joseph Blvd. Gatineau, Quebec K1A 0H3 Fax (819) 953-5053 Note: Website addresses mentioned in this document may have changed or references cited may have been removed from websites since the publication of the document. -

Transforming Lives and Advancing Economic Opportunity: EPA's
TRANSFORMING LIVES and ADVANCING ECONOMIC OPPORTUNITY: EPA’s Environmental Workforce Development and Job Training Program Preparing Unemployed and Underemployed Residents of Waste- Impacted Communities for Full-time Environmental Careers United States Environmental Protection Agency This page is intentionally left blank. B TRANSFORMING LIVES AND ADVANCING ECONOMIC OPPORTUNITY Contents Introduction ..................................................................................1 Superfund Site Cleanup ...............................................................5 • St. Louis Community College, Missouri ...........................................5 • Cypress Mandela Training Center, California ................................8 Solid Waste Management .........................................................13 • Zender Environmental Health and Research Group, Alaska ........13 • Northwest Regional Workforce Investment Board, Connecticut ...16 Wastewater Management ...........................................................20 • Rose State College, Oklahoma .....................................................20 • OAI, Inc. — Greencorps Chicago, Illinois .....................................22 Emergency Planning and Response ..........................................27 • Florida State College at Jacksonville, Florida ..............................27 • The Fortune Society, New York ....................................................30 Renewable Energy Installation ...................................................34 • City of Richmond, California -

Identifying Materials, Recipes and Choices: Some Suggestions for the Study of Archaeological Cupels
IDENTIFYING MATERIALS, RECIPES AND CHOICES: SOME SUGGESTIONS FOR THE STUDY OF ARCHAEOLOGICAL CUPELS Marcos Martinón-Torres – UCL Institute of Archaeology, London, United Kingdom Thilo Rehren – UCL Institute of Archaeology, London, United Kingdom Nicolas Thomas – INRAP and Université Paris I, Panthéon-Sorbonne, France Aude Mongiatti– UCL Institute of Archaeology, London, United Kingdom ABSTRACT Used cupels are increasingly identified in archaeological assemblages related to coin minting, alchemy, assaying and goldsmithing across the world. However, notwithstanding some valuable studies, the informative potential of cupellation remains is not always being exploited in full. Here we present a review of past and ongoing research on cupels, involving analytical studies, experiments and historical enquiry, and suggest some strategies for more productive future work. The archaeological case studies discussed are medieval and later assemblages from France (Pymont and Montbéliard) and Austria (Oberstockstall and Kapfenberg), which have been analysed using optical microscopy, SEM-EDS, ED-XRF, WD-EPMA and ICP-AES. Using suitable analytical and data processing methodologies, it is possible to obtain an insight into the metallurgical processes carried out in cupels, and the knowledge and skill of the craftspeople involved. Furthermore, we can also discern the specific raw materials used for manufacturing the cupels themselves, including varying mixtures of bone and wood ash. The variety of cupel-making recipes raises questions as to the versatility of craftspeople and the material properties and performance of different cupels. Can we assess the efficiency of different cupels? Are these variations the results of different technological traditions, saving needs or peculiar perceptions of matter? KEYWORDS Lead, silver, cupellation, fire assay, technological choice, bone ash, wood ash INTRODUCTION Cupellation is a high-temperature oxidising reaction aimed at refining noble metals. -
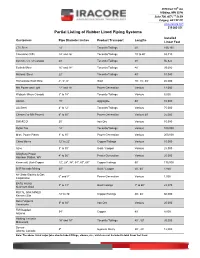
Lined Piping List.Xlsx
3516 East 13th Ave Hibbing, MN 55746 Suite 700, 407-2nd St SW Calgary, AB T2P 2Y3 www.iracore.com 218-262-5211 Partial Listing of Rubber Lined Piping Systems Installed Customers Pipe Diameter Inches Product Transport Lengths Linear Feet LTV Steel 14” Taconite/Tailings 40’ 155,160 Cleveland Cliffs 24” and 26” Taconite/Tailings 20’ to 40’ 58,310 Iron Ore Co. of Canada 20’ Taconite/Tailings 38’ 56,824 Eveleth Mine 16” and 18” Taconite/Tailings 40’ 35,000 National Steel 22” Taconite/Tailings 40’ 51,040 Homestake Gold Mine 4”, 5”, 6” Gold 10’, 20’, 40’ 45,000 MN Power and Light 12” and 18” Power Generation Various 12,000 Wabush Mines Canada 3” to 18” Taconite/Tailings Various 5,000 Unimin 10” Aggragate 40’ 10,000 US Steel 4” to 12” Taconite/Tailings Various 75,000 Cheme (for MN Power) 6” to 30” Power Generation Various 20’ 25,000 SMARCO 20” Iron Ore Various 10,000 Butler Tac 14” Taconite/Tailings Various 100,000 Misc. Power Plants 3” to 30” Power Generation Various 200,000 Chino Mines 12” to 22” Copper/Tailings Various 10,000 Azco 3” to 30” Gold / Copper Various 25,000 Allegheny Power 4” to 36” Power Generation Various 25,000 Harrison Station, WV Kennecott Utah Copper 12”, 28”, 38”, 54”, 60”, 66” Copper/Tailings 60’ 175,000 BHP Nevada Mining 30” Gold / Copper 50’, 60’ 7,850 NY State Electric & Gas 6” and 8” Power Generation Various 1,000 Corporation BATU HIJAU 3” to 44” Gold/Tailings 1” to 60’ 23,970 Newmont Gold ROYAL OAK MINES 24” to 36” Copper/Tailings 40’, 60’ 60,000 Kemess Site Duro Felguera 3" to 30" Iron Ore Various 20,000 Venezuela FMI Bagdad 34" Copper 40’ 8,000 Arizona Hibbing Taconite 16" and 18" Taconite/Tailings 40' , 50' 35,000 Minnesota Suncor 8" Gypsum Slurry 20' , 40’ 12,000 Alberta, Canada Note: The above listed major jobs also included fittings, elbows, etc., which are not included in total linear feet lined. -

Lecture 12 Converter Steelmaking Practice & Combined Blowing
Lecture 12 Converter Steelmaking Practice & combined blowing Contents: Refining of hot metal Composition and temperature during the blow Physico-chemical interactions Developments in Top blown steelmaking practice Concept of bottom stirring in top blowing Top blowing attributes Characteristic feature of converter steelmaking Environmental issues in oxygen steelmaking Causes of high turnover rates of BOF Key words: Top blown steelmaking, combined blowing, bottom stirring, hot metal refining Refining of hot metal After the previous heat is tapped and slag is drained, lining is inspected. Scrap and hot metal are charged. Converter is tilted into the vertical position and the lance is lowered in the vessel to start the blowing. Selection of the starting lance distance is such that the concentration of the force at the bath level should not cause ejection of tiny iron particles (sparking) and at the same time maximum bath surface area is covered by the oxygen jet. The starting lance distance(Xi) for specific oxygen blowing Nm 3 3 rate ton ×min can be calculated by 1.04 Xi = 0.541(db) db is bath diameter in meter. For 150 Tons converter, db = 4.87 m and Xi = 2.8 m, when oxygen flow rate in approximately 450 Nm3/min. Initially oxygen is blown soft by keeping lance distance higher to promote slag formation and to avoid ejection of small particles, because hot metal is not covered by slag. Lime may be added either at the beginning of the blow or in portion during the blow. Oxygen is blown for nearly 15-20 minutes by progressively decreasing the lance distance such that slag foaming remains under control and oxidation reactions occur uninterruptedly. -

Analysis of 999.9 Fine Gold by the Fire Assay Method and Common Sources of Error
Analysis of 999.9 fine gold by the fire assay method and common sources of error Dippal Manchanda MSc CSci CChem FRSC Technical Director & Chief Assayer The LBMA Assaying & Refining Conference London 2017 Chief Sources of Error The majority of errors in the fire assay operation comes from three sources: 1. Imperfection in even the finest balance. 2. Non-matching matrices i.e. differences in composition between the controlling proof assay sample and the alloy under examination. 3. Variations in temperature in different parts of the cupellation muffle. Other sources of error depend upon the skill of the worker who prepares the cupelled buttons for parting. We will identify these sources of errors and discuss ways to minimise them. The LBMA Assaying & Refining Conference London 2017 Two Pan Mechanical Balance vs Electronic Balance The LBMA Assaying & Refining Conference London 2017 Accuracy vs Weight Test Method Recommendation Why??? Weighing step. 999.9 fine gold - always weigh 500mg in Why 500mg? quadruplicate. Initial Fineness Final wt. (mg) Final Wt. (mg) Fineness Diff. in wt. (ppt.) [of 999.9 fine] [Say 0.01 mg error (ppt.) fineness (mg) occurred due to (+ side} any reason] 100 999.9 99.99 100.000 1000.00 0.1 ppt 250 999.9 249.975 249.985 999.94 0.04 ppt 500 999.9 499.95 499.960 999.92 0.02 ppt Higher the weight, better will be the accuracy The LBMA Assaying & Refining Conference London 2017 Silver to Gold Ratio Literature search reveals Optimum ratio??? Higher the silver content, lower will be the What is the optimum Ag: Au absorption loss during cupellation ratio? 1. -

Kennecott and Utah's Air Quality
Utah October 2018 kennecott.com/air-quality Kennecott and Utah’s air quality A closer look at winter inversion Wasatch Front Utah continues to have wintertime air quality problems During the winter, Kennecott shuts down its power despite decades of air quality regulations on companies plant reducing the amount of fine particulate emissions like Rio Tinto’s Kennecott Utah Copper. Utah meets the to 3.8 percent of a typical winter day. Additionally, EPA’s annual standard for fine particulate emissions (PM2.5) Kennecott has been actively working to decrease throughout the year. However, the state struggles to meet emissions from the other sources that contribute to the 24-hour standard during the winter inversion season, inversions, such as transportation and area sources. on average about 20-days per year. Though it will take the entire community working According to the Utah Division of Air Quality, Kennecott together to be successful, Kennecott is committed is responsible for 4.4 percent of the annual fine particulate to doing its part. emissions in the Salt Lake air shed. Kennecott’s impact on the valley’s air, especially during an inversion is To learn more, take a closer look at even less. kennecott.com/air-quality. From ore to more: our work makes modern life possible. Cu October 2018 kennecott.com/air-quality Kennecott Utah Copper Kennecott’s contribution to Utah’s air quality 6390 ft. 6390 aboveft. sea6390 level ft. above sea level above sea level Bingham Canyon Mine 6390Bingham ft. CanyonBingham Mine Canyon Mine 5550above sea f levelt. elevation 5550elevation aboveft. -

Kennecott Utah Copper Corporation
Miningmining BestPractices Plant-Wide Assessment Case Study Industrial Technologies Program Kennecott Utah Copper Corporation: Facility Utilizes Energy Assessments to Identify $930,000 in Potential Annual Savings BENEFITS • Identified potential annual cost savings of $930,000 Summary • Identified potential annual savings of Kennecott Utah Copper Corporation (KUCC) used targeted energy assessments in the smelter 452,000 MMBtu in natural gas and refinery at its Bingham Canyon Mine, near Salt Lake City, Utah, to identify projects to • Found opportunities to reduce maintenance, conserve energy and improve production processes. By implementing the projects identified repair costs, waste, and environmental during the assessment, KUCC could realize annual cost savings of $930,000 and annual energy emissions savings of 452,000 million British thermal units (MMBtu). The copper smelting and refining • Found opportunities to improve industrial facilities were selected for the energy assessments because of their energy-intensive processes. Implementing the projects identified in the assessments would also reduce maintenance, hygiene and safety repair costs, waste, and environmental emissions. One project would use methane gas from • Identified ways to improve process an adjacent municipal dump to replace natural gas used to heat the refinery electrolyte. throughput Public-Private Partnership • Identified a potential payback period of less than 1 year for all projects combined The U.S. Department of Energy's (DOE) Industrial Technologies Program (ITP) cosponsored the assessment. DOE promotes plant-wide energy-efficiency assessments that will lead to improvements in industrial energy efficiency, productivity, and global competitiveness, while reducing waste and environmental emissions. In this case, DOE contributed $100,000 of the total $225,000 assessment cost.