Developmental Angiogenesis: Quail Embryonic Vasculature
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cardiovascular System Heart Development Cardiovascular System Heart Development
Cardiovascular System Heart Development Cardiovascular System Heart Development In human embryos, the heart begins to beat at approximately 22-23 days, with blood flow beginning in the 4th week. The heart is one of the earliest differentiating and functioning organs. • This emphasizes the critical nature of the heart in distributing blood through the vessels and the vital exchange of nutrients, oxygen, and wastes between the developing baby and the mother. • Therefore, the first system that completes its development in the embryo is called cardiovascular system. https://www.slideshare.net/DrSherifFahmy/intraembryonic-mesoderm-general-embryology Mesoderm is one of the three • Connective tissue primary germ layers that • Smooth and striated muscle • Cardiovascular System differentiates early in • Kidneys development that collectively • Spleen • Genital organs, ducts gives rise to all subsequent • Adrenal gland cortex tissues and organs. The cardiovascular system begins to develop in the third week of gestation. Blood islands develop in the newly formed mesoderm, and consist of (a) a central group of haemoblasts, the embryonic precursors of blood cells; (b) endothelial cells. Development of the heart and vascular system is often described together as the cardiovascular system. Development begins very early in mesoderm both within (embryonic) and outside (extra embryonic, vitelline, umblical and placental) the embryo. Vascular development occurs in many places. • Blood islands coalesce to form a vascular plexus. Preferential channels form arteries and veins. • Day 17 - Blood islands form first in the extra-embryonic mesoderm • Day 18 - Blood islands form next in the intra-embryonic mesoderm • Day 19 - Blood islands form in the cardiogenic mesoderm and coalesce to form a pair of endothelial heart tubes Development of a circulation • A circulation is established during the 4th week after the myocardium is differentiated. -

Congenital Abnormalities of the Aortic Arch: Revisiting the 1964 Stewart
Cardiovascular Pathology 39 (2019) 38–50 Contents lists available at ScienceDirect Cardiovascular Pathology Review Article Congenital abnormalities of the aortic arch: revisiting the 1964 ☆ Stewart classification Shengli Li a,⁎,HuaxuanWena,MeilingLianga,DandanLuoa, Yue Qin a,YimeiLiaoa, Shuyuan Ouyang b, Jingru Bi a, Xiaoxian Tian c, Errol R. Norwitz d,GuoyangLuoe,⁎⁎ a Department of Ultrasound, Shenzhen Maternity & Child Healthcare Hospital, Affiliated to Southern Medical University, Shenzhen, 518028, China b Department of Laboratory Medicine, Shenzhen Maternity & Child Healthcare Hospital, Affiliated to Southern Medical University, Shenzhen, 518028, China c Department of Ultrasound, Maternity & Child Healthcare Hospital of Guangxi Zhuang Autonomous Region, Nanning, Guangxi, 538001, China d Department of Obstetrics & Gynecology, Tufts University School of Medicine, Boston, MA 02111 e Department of Obstetrics & Gynecology, Howard University, College of Medicine, Washington, DC 20060, USA article info abstract Article history: The traditional classification of congenital aortic arch abnormalities was described by James Stewart and col- Received 12 June 2018 leagues in 1964. Since that time, advances in diagnostic imaging technology have led to better delineation of Received in revised form 27 November 2018 the vasculature anatomy and the identification of previously unrecognized and unclassified anomalies. In this Accepted 28 November 2018 manuscript, we review the existing literature and propose a series of modifications to the original Stewart -

Development of the Vascular System in Five to Twenty-One
THE DEVELOPMENT OF THE VASCULAR SYSTEM IN FIVE TO TWtNTY-ONE SOMITE DOG EMBRYOS by ELDEN WILLIAM MARTIN B, S., Kansas State College of Agriculture and ADolied Science, 195>U A THESIS submitted in partial fulfillment of the requirements for the degree MASTER OF SCIENCE Department of Zoology KANSAS STATV: COLLEGE OF AGRICULTURE AND A PLIED SCIENCE 1958 LP TH Ooco/*>*Tv TABLE OF CONTENTS INTRO IXJ CTION AND LITERATURE REVIEW 1 MATERIALS AND METHODS ^ OBSERVATIONS 6 Five-Somi te Stag© . 6 Seven-Somite Stage 8 Eight-Somite Stage 9 Ten- and bleven-Somite Stage 12 Twe 1 ve-Somi te Stage • \\i Fifteen-Somite Stage 18 Seventeen-Somite Stage 21 Eighteen-Somite Stage 2$ Twenty- and Twenty- one -Somite Stage 27 INTERPRETATIONS AND DISCUSSION 30 Vasculogenesis • 30 Cardiogenesis 33 The Origin and Development of Arteries \ 3lj. Aortic Arches •••« 3I4. Cranial Arterie s ...•• 36 The Dorsal Aorta 37 Intersegmental AAteries 39 Vertebral Arteries 39 Vitelline Arteries }±q The Allantoic Artery \±\ Ill IITERPRETATION AND DISCUSSION (Contd.) The Origin and Development of Veins •• kl The Anterior Cardinal Veins . I4.I Posterior Cardinal Veins k2 Umbilical Veins U3 Common Cardinal Veins kh Interconnecting Vessels Ui> SUMMARY kl LITERA°URE CITED $1 ACKNOWLEDGMENTS 53 APPENDIX 5U HTmDUCTIOW AND LITFRATORF. rfvibw While the dog has been employed extensively as a labora- tory animal in various fields of scientific endeavour, the use of this animal in embryology has been neglected. As a con- sequence, the literature on the circulatory system of the dog was represented only by an unpublished thesis by Duffey (3) on oardlogenesis and the first heart movements. -

Cardiovascular System Note: the Cardiovascular System Develops Early (Week 3), Enabling the Embryo to Grow Beyond the Short
Lymphatics: Lymph vessel formation is similar to blood angiogenesis. Lymphatics begin as lymph sacs in three regions: jugular (near brachiocephalic veins); cranial abdominal (future cysterna chyla); and iliac region. Lym- phatic vessels (ducts) form as outgrowths of the sacs. mesenchyme Lymph nodes are produced by localized mesoder- sinusoid lymph duct lumen mal invaginations that partition the vessel lumen into sinu- soids. The mesoderm develops a reticular framework within which mesodermal lymphocytes accumulate. The spleen and hemal nodes (in ruminants) invagination develop similar to the way lymph nodes develop. Lymph Node Formation Prior to birth, fetal circulation is designed for an in utero aqueous environment where the pla- centa oxygenates fetal blood. Suddenly, at birth... Three In-Utero Adjustments ductus Stretching and constriction of arteriosus umbilical arteries shifts fetal blood flow aortic arch from the placenta to the fetus. Reduced pulmonary trunk L atrium venous return through the (left) umbili- foramen ovale R cal vein and ductus venosus allows the atrium latter to gradually close (over a period caudal vena cava of days). Bradykinin released by expand- ductus venosus ing lungs and increased oxygen concen- tration in blood triggers constriction of aorta the ductus arteriosus which, over two liver months, is gradually converted to a fibrous structure, the ligamentum arte- umbilical v. riosum. portal v. The increased blood flow to the lungs and then to the left atrium equalizes pres- sure in the two atria, resulting in closure umbilical aa. of the foramen ovale that eventually grows permanent. 29 The cardiogenic area, the place where the embryonic heart originates, is located . -

6 Development of the Great Vessels and Conduction Tissue
Development of the Great Vessels and Conduc6on Tissue Development of the heart fields • h:p://php.med.unsw.edu.au/embryology/ index.php?6tle=Advanced_-_Heart_Fields ! 2 Septa6on of the Bulbus Cordis Bulbus Cordis AV Canal Ventricle Looking at a sagital sec6on of the heart early in development the bulbus cordis is con6nuous with the ventricle which is con6nuous with the atria. As the AV canal shiOs to the right the bulbus move to the right as well. Septa6on of the Bulbus Cordis A P A P The next three slides make the point via cross sec6ons that the aorta and pulmonary arteries rotate around each other. This means the septum between them changes posi6on from superior to inferior as well. Septa6on of the Bulbus Cordis P A A P Septa6on of the Bulbus Cordis P A P A Migra6on of neural crest cells Neural crest cells migrate from the 3ed, 4th and 6th pharyngeal arches to form some of the popula6on of cells forming the aor6copulmonary septum. Septa6on of the Bulbus Cordis Truncal (Conal) Swellings Bulbus Cordis The cardiac jelly in the region of the truncus and conus adds the neural crest cells and expands as truncal swellings. Septa6on of the Bulbus Cordis Aorticopulmonary septum These swellings grow toward each other to meet and form the septum between the aorta and pulmonary artery. Aorta Pulmonary Artery Septa6on of the Bulbus Cordis Anterior 1 2 3 1 2 3 The aor6copulmonary septum then rotates as it moves inferiorly. However, the exact mechanism for that rota6on remains unclear. Septa6on of the Bulbus Cordis Aorta Pulmonary Artery Conal Ridges IV Foramen Membranous Muscular IV Endocarial Septum Interventricular Cushion Septum However, the aor6copulmonary septum must form properly for the IV septum to be completed. -

Human Placenta Is a Potent Hematopoietic Niche Containing Hematopoietic Stem and Progenitor Cells Throughout Development
Cell Stem Cell Article Human Placenta Is a Potent Hematopoietic Niche Containing Hematopoietic Stem and Progenitor Cells throughout Development Catherine Robin,1,5 Karine Bollerot,1,5 Sandra Mendes,1 Esther Haak,1 Mihaela Crisan,1 Francesco Cerisoli,1 Ivoune Lauw,1 Polynikis Kaimakis,1 Ruud Jorna,1 Mark Vermeulen,3 Manfred Kayser,3 Reinier van der Linden,1 Parisa Imanirad,1 Monique Verstegen,2 Humaira Nawaz-Yousaf,1 Natalie Papazian,2 Eric Steegers,4 Tom Cupedo,2 and Elaine Dzierzak1,* 1Erasmus MC Stem Cell Institute, Department of Cell Biology 2Department of Hematology 3Department of Forensic Molecular Biology 4Department of Obstetrics and Gynecology Erasmus University Medical Center, 3000 CA Rotterdam, the Netherlands 5These authors contributed equally to this work *Correspondence: [email protected] DOI 10.1016/j.stem.2009.08.020 SUMMARY becomes hematopoietic. The emergence of multipotent progen- itors and HSCs, organized in clusters of cells closely adherent to Hematopoietic stem cells (HSCs) are responsible for the ventral wall of the dorsal aorta, starts at day 27 in the devel- the life-long production of the blood system and are oping splanchnopleura/AGM region (Tavian et al., 1996, 1999, pivotal cells in hematologic transplantation thera- 2001). Starting at day 30, the first erythroid progenitors (BFU- pies. During mouse and human development, the E, burst forming unit erythroid) are found in the liver, with multi- first HSCs are produced in the aorta-gonad-meso- lineage hematopoietic progenitors (CFU-Mix or -GEMM; colony nephros region. Subsequent to this emergence, forming unit granulocyte, erythroid, macrophage, megakaryo- cyte) appearing in this tissue at week 13 (Hann et al., 1983). -
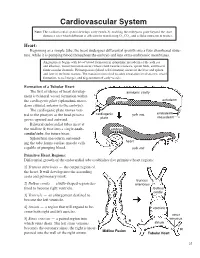
Cardiovascular System Note: the Cardiovascular System Develops Early (Week-3), Enabling the Embryo to Grow Beyond the Short
Cardiovascular System Note: The cardiovascular system develops early (week-3), enabling the embryo to grow beyond the short distances over which diffusion is efficient for transferring 2O , CO2, and cellular nutrients & wastes. Heart: Beginning as a simple tube, the heart undergoes differential growth into a four chambered struc- ture, while it is pumping blood throughout the embryo and into extra-embryonic membranes. Angiogenesis begins with blood island formation in splanchnic mesoderm of the yolk sac and allantois. Vessel formation occurs when island vesicles coalesce, sprout buds, and fuse to form vascular channels. Hematopoiesis (blood cell formation) occurs in the liver and spleen and later in the bone marrow. The transition from fetal to adult circulation involves new vessel formation, vessel merger, and degeneration of early vessels. Formation of a Tubular Heart: The first evidence of heart develop- amnionic cavity ment is bilateral vessel formation within ectoderm the cardiogenic plate (splanchnic meso- embryo derm situated anterior to the embryo). The cardiogenic plate moves ven- tral to the pharynx as the head process cardiogenic yolk sac endoderm mesoderm grows upward and outward. plate Bilateral endocardial tubes meet at the midline & fuse into a single endo- embryo cardial tube, the future heart. Splanchnic mesoderm surround- ing the tube forms cardiac muscle cells heart capable of pumping blood. yolk sac Primitive Heart Regions: Differential growth of the endocardial tube establishes five primitive heart regions: 1] Truncus arteriosus — the output region of the heart. It will develop into the ascending aorta and pulmonary trunk. truncus 2] Bulbus cordis — a bulb-shaped region des- arteriosus tined to become right ventricle. -

Aortic Arches
Human Embryology: Heart Development II Kimara L. Targoff, M.D. Division of Pediatric Cardiology, Columbia University Medical Center Developmental Genetics Program, Skirball Institute, NYU School of Medicine Human Vascular Development • Overview • Aortic Arch Development • Arterial Vascular Development • Venous System Development • Lymphatic Development • Transition from Fetal to Post-Natal Circulation Development of the Arterial and Venous Systems Cranial Ends of the Dorsal Aortae Form a Dorsoventral Loop: The First Aortic Arch Aortic Arches Arise in a Craniocaudal Sequence Surrounding the Pharynx Aortic Arches Give Rise to Important Head, Neck, and Upper Thorax Vessels Aortic Arch Development in the Chick Embryo Fgf8 is Required for Pharyngeal Arch Development in Mouse Abu-Issa, R. et al., Development 2002. Cardiovascular and Thymic Defects in Tbx1 Hypomorphic Mutant Neonates Hu, T. et al., Development 2004. Aortic Arch Development Dorsal aorta 1 2 3 Ventral aorta 4 5 6 7 iseg Harsh Thaker Aortic Arch Development Dorsal aorta 1 2 3 Ventral aorta 4 5 6 7 iseg Harsh Thaker Aortic Arch and Derivatives 3 3 4 4 7 iseg 6 7 iseg 6 Aortic sac Truncus arteriosus Harsh Thaker Aortic Arch and Derivatives 3 3 4 4 7 iseg 6 7 iseg Harsh Thaker Aortic Arch and Derivatives 3 3 4 7 iseg 4 7 iseg 6 Harsh Thaker Aortic Arch and Derivatives RCC LCC RSC LSC BCA DA Harsh Thaker Recurrent Laryngeal Nerves RCC LCC RSC LSC BCA DA Harsh Thaker Defects in Normal Regression of the Arterial System Lead to Vascular Anomalies • Double Aortic Arch – Failure of the -

The Allantois and Chorion, When Isolated Before Circulation Or Chorio-Allantoic Fusion, Have Hematopoietic Potential
Dartmouth College Dartmouth Digital Commons Open Dartmouth: Published works by Dartmouth faculty Faculty Work 11-2006 The Allantois and Chorion, when Isolated before Circulation or Chorio-Allantoic Fusion, have Hematopoietic Potential Brandon M. Zeigler Dartmouth College Daisuke Sugiyama Dartmouth College Michael Chen Dartmouth College Yalin Guo Dartmouth College K. M. Downs University of Wisconsin-Madison See next page for additional authors Follow this and additional works at: https://digitalcommons.dartmouth.edu/facoa Part of the Biochemistry Commons, Cell and Developmental Biology Commons, and the Genetics Commons Dartmouth Digital Commons Citation Zeigler, Brandon M.; Sugiyama, Daisuke; Chen, Michael; Guo, Yalin; Downs, K. M.; and Speck, N. A., "The Allantois and Chorion, when Isolated before Circulation or Chorio-Allantoic Fusion, have Hematopoietic Potential" (2006). Open Dartmouth: Published works by Dartmouth faculty. 734. https://digitalcommons.dartmouth.edu/facoa/734 This Article is brought to you for free and open access by the Faculty Work at Dartmouth Digital Commons. It has been accepted for inclusion in Open Dartmouth: Published works by Dartmouth faculty by an authorized administrator of Dartmouth Digital Commons. For more information, please contact [email protected]. Authors Brandon M. Zeigler, Daisuke Sugiyama, Michael Chen, Yalin Guo, K. M. Downs, and N. A. Speck This article is available at Dartmouth Digital Commons: https://digitalcommons.dartmouth.edu/facoa/734 RESEARCH ARTICLE 4183 Development 133, 4183-4192 (2006) doi:10.1242/dev.02596 The allantois and chorion, when isolated before circulation or chorio-allantoic fusion, have hematopoietic potential Brandon M. Zeigler1, Daisuke Sugiyama1,*, Michael Chen1, Yalin Guo1, Karen M. Downs2,† and Nancy A. -

Vascular Endothelial Tyrosine Phosphatase (VE-PTP)-Null Mice Undergo Vasculogenesis but Die Embryonically Because of Defects in Angiogenesis
Vascular endothelial tyrosine phosphatase (VE-PTP)-null mice undergo vasculogenesis but die embryonically because of defects in angiogenesis Melissa G. Dominguez, Virginia C. Hughes, Li Pan, Mary Simmons, Christopher Daly, Keith Anderson, Irene Noguera-Troise, Andrew J. Murphy, David M. Valenzuela, Samuel Davis, Gavin Thurston, George D. Yancopoulos*, and Nicholas W. Gale* Regeneron Pharmaceuticals, Inc., 777 Old Saw Mill River Road, Tarrytown, NY 10591 Contributed by George D. Yancopoulos, December 28, 2006 (sent for review December 21, 2006) Development of the vascular system depends on the highly coordi- vascular endothelial PTP (VE-PTP) (4). VE-PTP, the mouse nated actions of a variety of angiogenic regulators. Several of these homologue of receptor-type human PTP- (5), has been shown to regulators are members of the tyrosine kinase superfamily, including associate with and dephosphorylate Tie2 (4) and VE-cadherin (6) VEGF receptors and angiopoietin receptors, Tie1 and Tie2. Tyrosine but was shown to not interact with VEGF-R2 (4). kinase signaling is counter-regulated by the activity of tyrosine To study the expression pattern in embryonic and adult tissues phosphatases, including vascular endothelial protein tyrosine phos- and to elucidate the functional role of VE-PTP in vascular devel- phatase (VE-PTP), which has previously been shown to modulate Tie2 opment, we generated mice in which the VE-PTP gene was activity. We generated mice in which VE-PTP is replaced with a replaced by LacZ, a high-resolution reporter gene. LacZ expression reporter gene. We confirm that VE-PTP is expressed in endothelium confirms that VE-PTP is expressed in both arterial and venous and also show that VE-PTP is highly expressed in the developing vascular endothelium in embryos, although more strongly in arterial outflow tract of the heart and later is expressed in developing heart vessels. -

Development of the Endocardium
Pediatr Cardiol DOI 10.1007/s00246-010-9642-8 RILEY SYMPOSIUM Development of the Endocardium Ian S. Harris • Brian L. Black Received: 7 January 2010 / Accepted: 17 January 2010 Ó The Author(s) 2010. This article is published with open access at Springerlink.com Abstract The endocardium, the endothelial lining of the cardiac crescent. We propose a third model that reconciles heart, plays complex and critical roles in heart develop- these two views and suggest future experiments that might ment, particularly in the formation of the cardiac valves resolve this question. and septa, the division of the truncus arteriosus into the aortic and pulmonary trunks, the development of Purkinje Keywords Endocardium Á Endothelial Á Heart fibers that form the cardiac conduction system, and the formation of trabecular myocardium. Current data suggest that the endocardium is a regionally specialized endothe- Overview of Cardiogenesis lium that arises through a process of de novo vasculogen- esis from a distinct population of mesodermal cardiogenic The cardiovascular system is the first organ system to form precursors in the cardiac crescent. In this article, we review and function in the vertebrate embryo. The heart forms recent developments in the understanding of the embryonic when regions of bilaterally symmetrical cardiac progenitor origins of the endocardium. Specifically, we summarize cells in the anterior lateral mesoderm fuse at the ventral vasculogenesis and specification of endothelial cells from midline to form a linear tube, which is continuous with the mesodermal precursors, and we review the transcriptional dorsal aorta anteriorly and the cardinal veins posteriorly pathways involved in these processes. We discuss the [5]. -

Aortic Arch Evolution
Aortic Arch Evolution The aortic arches are the blood vessels that supply the pharyngeal arches, and they serve as a communication between the ventral and dorsal aortae. The ventral aorta is the main artery into which the truncus arteriosus leads. It bifurcates into left and right vessels which extend forward as the paired external carotids , whilst the paired dorsal aortae extend forward as the internal carotids. They are paired, serving the left and right pharyngeal regions which are basically similar in number and disposition in different vertebrates during the embryonic stages. The appearance of six aortic arches during the embryonic development of living gnathostomes suggests that this is the ancestral pattern. However, as we have seen, the actual adult anatomy can be quite varied among different species. Basic plan of Aortic arches • Continuous vessels. • Typical number 6 pairs (7 in primitive shark, many in cyclostome). • Developed in a similar fashion (anterior to posterior). • First (I): Mandibular arch (proceed upword on either side of pharynx). • Second (II): Hyoid arch. • Rest: III (1 st branchial), IV (2 nd branchial), V (3 rd branchial), VI (4 th branchial) aortic arch. Modification of aortic arches in different vertebrates during evolution is based on following: Scheme of Evolution In Petromyzon, there are 7 pairs of aortic arches. In other cyclostomes these vary from 6 pairs in Myxine and 15 pairs in Eptatretus. Primitive fishes, represented by sharks, have six paired gill arches. In teleosts, the gill arch arteries are reduced to form four pairs in the caudal branchial arches. First pair (mandibular) and second pair (hyoidean) are lost.