Antiarrhythmic Medications for Cardioversion and Maintenance Of
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Table 2. 2012 AGS Beers Criteria for Potentially
Table 2. 2012 AGS Beers Criteria for Potentially Inappropriate Medication Use in Older Adults Strength of Organ System/ Recommendat Quality of Recomm Therapeutic Category/Drug(s) Rationale ion Evidence endation References Anticholinergics (excludes TCAs) First-generation antihistamines Highly anticholinergic; Avoid Hydroxyzin Strong Agostini 2001 (as single agent or as part of clearance reduced with e and Boustani 2007 combination products) advanced age, and promethazi Guaiana 2010 Brompheniramine tolerance develops ne: high; Han 2001 Carbinoxamine when used as hypnotic; All others: Rudolph 2008 Chlorpheniramine increased risk of moderate Clemastine confusion, dry mouth, Cyproheptadine constipation, and other Dexbrompheniramine anticholinergic Dexchlorpheniramine effects/toxicity. Diphenhydramine (oral) Doxylamine Use of diphenhydramine in Hydroxyzine special situations such Promethazine as acute treatment of Triprolidine severe allergic reaction may be appropriate. Antiparkinson agents Not recommended for Avoid Moderate Strong Rudolph 2008 Benztropine (oral) prevention of Trihexyphenidyl extrapyramidal symptoms with antipsychotics; more effective agents available for treatment of Parkinson disease. Antispasmodics Highly anticholinergic, Avoid Moderate Strong Lechevallier- Belladonna alkaloids uncertain except in Michel 2005 Clidinium-chlordiazepoxide effectiveness. short-term Rudolph 2008 Dicyclomine palliative Hyoscyamine care to Propantheline decrease Scopolamine oral secretions. Antithrombotics Dipyridamole, oral short-acting* May -

Vernakalant Versus Ibutilide for Immediate Conversion of Recent-Onset Atrial Fibrillation Vogiatzis I, Papavasiliou E, Dapcevitch I, Pittas S, Koulouris E
HIPPOKRATIA 2017, 21, 2: 67-73 RESEARCH ARTICLE Vernakalant versus ibutilide for immediate conversion of recent-onset atrial fibrillation Vogiatzis I, Papavasiliou E, Dapcevitch I, Pittas S, Koulouris E Department of Cardiology, General Hospital of Veroia, Veroia, Greece Abstract Background: The pharmacological cardioversion of recent-onset atrial fibrillation (AF) is a challenge for the clinician. The aim of the study was to compare the efficacy, the safety, and the overall cost of intravenous (iv) administration of vernakalant, which is a relatively new atrial-selective antiarrhythmic agent, versus ibutilide, in cardioversion of recent- onset AF. Methods: We enrolled in this study 78 patients (56 men, 22 women; mean age 63.72 ± 6.67 years) who presented with recent-onset AF. Cardioversion was attempted in 36 patients (group A: 24 men, 12 women; mean age 62.44 ± 7.24 years) by iv administration of vernakalant (3 mg/kg over 10 min and if needed after 15 min, a second dose 2 mg/kg over 10 min) while in 42 patients (group B: 32 men, 10 women; mean age 64.81 ± 6 years) iv ibutilide was administered (1 mg over 10 min and if needed after 10 min, a second dose 1 mg over 10 min). Results: AF was successfully converted in 52.78 % of (n =19) patients of group A vs 52.38 % of (n =22) patients of group B (p =0.58), with an average time of conversion 11.8 ± 4.3 min for group A patients vs 33.9 ± 20.25 min for group B patients (p <0.0001). The average length of hospital stay for patients of group A was 17.64 ± 15.96 hours vs 41.09 ± 17.6 hours for patients of Group B (p <0.0001). -

Arrhythmias in the Intensive Care Patient Hans-Joachim Trappe, Bodo Brandts and Peter Weismueller
Arrhythmias in the intensive care patient Hans-Joachim Trappe, Bodo Brandts and Peter Weismueller Purpose of review ventricular fibrillation. The use of automatic external Atrial fibrillation, atrial flutter, AV–nodal reentry tachycardia defibrillators by basic life support ambulance providers or first with rapid ventricular response, atrial ectopic tachycardia, and responder in early defibrillation programs has been associated preexcitation syndromes combined with atrial fibrillation or with a significant increase in survival rates. Drugs such as ventricular tachyarrhythmias are typical arrhythmias in intensive lidocaine, procainamide, sotalol, amiodarone, or magnesium care patients. Most frequently, the diagnosis of the underlying were recommended for treatment of ventricular arrhythmia is possible from the physical examination, the tachyarrhythmias in intensive care patients. Amiodarone is a response to maneuvers or drugs, and the 12-lead surface highly efficacious antiarrhythmic agent for many cardiac electrocardiogram. In all patients with unstable hemodynamics, arrhythmias, ranging from atrial fibrillation to malignant immediate DC-cardioversion is indicated. Conversion of atrial ventricular tachyarrhythmias, and seems to be superior to other fibrillation to sinus rhythm is possible using antiarrhythmic antiarrhythmic agents. drugs. Amiodarone has a conversion rate in atrial fibrillation of up to 80%. However, caution in the use of short-term Keywords administration of intravenous amiodarone in critically ill patients atrial fibrillation, -

Properties and Units in Clinical Pharmacology and Toxicology
Pure Appl. Chem., Vol. 72, No. 3, pp. 479–552, 2000. © 2000 IUPAC INTERNATIONAL FEDERATION OF CLINICAL CHEMISTRY AND LABORATORY MEDICINE SCIENTIFIC DIVISION COMMITTEE ON NOMENCLATURE, PROPERTIES, AND UNITS (C-NPU)# and INTERNATIONAL UNION OF PURE AND APPLIED CHEMISTRY CHEMISTRY AND HUMAN HEALTH DIVISION CLINICAL CHEMISTRY SECTION COMMISSION ON NOMENCLATURE, PROPERTIES, AND UNITS (C-NPU)§ PROPERTIES AND UNITS IN THE CLINICAL LABORATORY SCIENCES PART XII. PROPERTIES AND UNITS IN CLINICAL PHARMACOLOGY AND TOXICOLOGY (Technical Report) (IFCC–IUPAC 1999) Prepared for publication by HENRIK OLESEN1, DAVID COWAN2, RAFAEL DE LA TORRE3 , IVAN BRUUNSHUUS1, MORTEN ROHDE1, and DESMOND KENNY4 1Office of Laboratory Informatics, Copenhagen University Hospital (Rigshospitalet), Copenhagen, Denmark; 2Drug Control Centre, London University, King’s College, London, UK; 3IMIM, Dr. Aiguader 80, Barcelona, Spain; 4Dept. of Clinical Biochemistry, Our Lady’s Hospital for Sick Children, Crumlin, Dublin 12, Ireland #§The combined Memberships of the Committee and the Commission (C-NPU) during the preparation of this report (1994–1996) were as follows: Chairman: H. Olesen (Denmark, 1989–1995); D. Kenny (Ireland, 1996); Members: X. Fuentes-Arderiu (Spain, 1991–1997); J. G. Hill (Canada, 1987–1997); D. Kenny (Ireland, 1994–1997); H. Olesen (Denmark, 1985–1995); P. L. Storring (UK, 1989–1995); P. Soares de Araujo (Brazil, 1994–1997); R. Dybkær (Denmark, 1996–1997); C. McDonald (USA, 1996–1997). Please forward comments to: H. Olesen, Office of Laboratory Informatics 76-6-1, Copenhagen University Hospital (Rigshospitalet), 9 Blegdamsvej, DK-2100 Copenhagen, Denmark. E-mail: [email protected] Republication or reproduction of this report or its storage and/or dissemination by electronic means is permitted without the need for formal IUPAC permission on condition that an acknowledgment, with full reference to the source, along with use of the copyright symbol ©, the name IUPAC, and the year of publication, are prominently visible. -

Safety and Efficacy of Ibutilide in Cardioversion of Atrial Flutter And
J Am Board Fam Med: first published as 10.3122/jabfm.2011.01.080096 on 5 January 2011. Downloaded from CLINICAL REVIEW Safety and Efficacy of Ibutilide in Cardioversion of Atrial Flutter and Fibrillation Madhuri Nair, MD, Lekha K. George, MD, and Santhosh K. G. Koshy, MD This article reviews the safety and efficacy of ibutilide for use in patients with atrial fibrillation and flut- ter. Ibutilide, a class III antiarrhythmic agent, is primarily used for conversion of atrial flutter and fi- brillation and is a good alternative to electrical cardioversion. Ibutilide has a conversion rate of up to 75% to 80% in recent-onset atrial fibrillation and flutter; the conversion rate is higher for atrial flutter than for atrial fibrillation. It is also safe in the conversion of chronic atrial fibrillation/flutter among patients receiving oral amiodarone therapy. Ibutilide pretreatment facilitates transthoracic defibrilla- tion and decreases the energy requirement of electrical cardioversion by both monophasic and biphasic shocks. Pretreatment with ibutilide before electrical defibrillation has a conversion rate of 100% com- pared with 72% with no pretreatment. Ibutilide is also safe and efficient in the treatment of atrial fibril- lation in patients who have had cardiac surgery, and in accessory pathway–mediated atrial fibrillation where the conversion rate of ibutilide is as high as 95%. There is up to a 4% risk of torsade de pointes and a 4.9% risk of monomorphic ventricular tachycardia. Hence, close monitoring in an intensive care unit setting is warranted during and at least for 4 hours after drug infusion. The anticoagulation strat- egy is the same as for any other mode of cardioversion.(J Am Board Fam Med 2011;24:86–92.) Keywords: Antiarrhythmics, Arrhythmia, Atrial Fibrillation, Cardiovascular Disorders, Cardioversion, Drug Ther- copyright. -

Efficacy and Safety of Ibutilide for the Cardioversion of Atrial Fibrillation: a Systematic Review and Meta- Analysis
Open Access Austin Surgery Case Reports Review Article Efficacy and Safety of Ibutilide for the Cardioversion of Atrial Fibrillation: A Systematic Review and Meta- Analysis Gong CC1, Tang Y2, Huang Y2 and Liu X2* 1Zhejiang University School of Medicine Children’s Abstract Hospital, China Background: Ibutilide has been approved for cardioversion of Atrial 2Department of Critical Care Medicine, the Affiliated Fibrillation (AF), but its side-effects include a high risk of torsade de pointes, Hospital of Guizhou Medical University, China besides, one recent meta-analysis showed ibutilide was inferior to vernakalant *Corresponding author: Liu X, Department of Critical for conversion (AF<7 days). Hence, the aim of this study is to evaluate the Care Medicine, the Affiliated Hospital of Guizhou Medical efficacy and safety of ibutilide for the cardioversion of AF within 90 days. University, China Methods: The Embase, PubMed, Web of Science, Cochrane Central Received: February 16, 2021; Accepted: March 17, databases and clinical trials.gov were comprehensively searched for relevant 2021; Published: March 24, 2021 studies from January 1991 to May 2020 using the keywords “ibutilide” and “atrial fibrillation”. Only Randomized Controlled Trials (RCTs) comparing ibutilide with placebo or other Anti-Arrhythmic Drugs (AADs) for the termination of AF (duration of AF ≤90 days) were included. The primary outcome was successful cardioversion in response to ibutilide versus placebo or other AADs within 4h. Related adverse events were defined as secondary outcomes. Results: A total of 1712 patients in 13 RCTs met the eligibility criteria. Four trials compared ibutilide to placebo; nine trials compared ibutilide to other active drugs. -
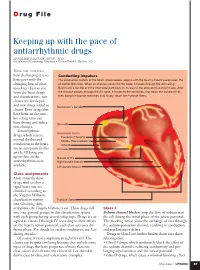
Keeping up with the Pace of Antiarrhythmic Drugs ANNMARIE PALATNIK, APN,BC, MSN Coordinator of Continuing Education • Virtua Health • Marlton, N.J
D rug File Keeping up with the pace of antiarrhythmic drugs ANNMARIE PALATNIK, APN,BC, MSN Coordinator of Continuing Education • Virtua Health • Marlton, N.J. HAVE YOU NOTICED how challenging it is to Conducting impulses keep pace with the The conduction system of the heart, shown below, begins with the heart’s natural pacemaker, the changing beat of phar- sinoatrial (SA) node. When an impulse leaves the SA node, it travels through the atria along macology? Just as you Bachmann’s bundle and the internodal pathways on its way to the atrioventricular (AV) node. After learn the latest drugs the impulse passes through the AV node, it travels to the ventricles, first down the bundle of His, and classifications, new then along the bundle branches and, finally, down the Purkinje fibers. classes are developed and new drugs added to Bachmann’s bundle classes. Even drugs that have been on the mar- ket a long time can have dosing and indica- SA node tion changes. Antiarrhythmic Internodal tracts drugs, which restore Posterior (Thorel’s) normal rhythm and Middle (Wenckebach’s) conduction to the heart, Anterior are no exception. In this AV node article, I’ll bring you up-to-date on the Bundle of His antiarrhythmics now Right bundle branch available. Left bundle branch Class assignments Most antiarrhythmic drugs used to slow a rapid heart rate are classified according to the Vaughn Williams classification system Purkinje fibers (see Classifying Anti- arrhythmics the Vaughn Williams way). These drugs fall Class I into four general groups in this classification system Sodium channel blockers stop the flow of sodium into with each group having several subgroups. -

Ventricular Tachycardia Drugs Versus Devices John Camm St
Cardiology Update 2015 Davos, Switzerland: 8-12th February 2015 Ventricular Arrhythmias Ventricular Tachycardia Drugs versus Devices John Camm St. George’s University of London, UK Imperial College, London, UK Declaration of Interests Chairman: NICE Guidelines on AF, 2006; ESC Guidelines on Atrial Fibrillation, 2010 and Update, 2012; ACC/AHA/ESC Guidelines on VAs and SCD; 2006; NICE Guidelines on ACS and NSTEMI, 2012; NICE Guidelines on heart failure, 2008; NICE Guidelines on Atrial Fibrillation, 2006; ESC VA and SCD Guidelines, 2015 Steering Committees: multiple trials including novel anticoagulants DSMBs: multiple trials including BEAUTIFUL, SHIFT, SIGNIFY, AVERROES, CASTLE- AF, STAR-AF II, INOVATE, and others Events Committees: one trial of novel oral anticoagulants and multiple trials of miscellaneous agents with CV adverse effects Editorial Role: Editor-in-Chief, EP-Europace and Clinical Cardiology; Editor, European Textbook of Cardiology, European Heart Journal, Electrophysiology of the Heart, and Evidence Based Cardiology Consultant/Advisor/Speaker: Astellas, Astra Zeneca, ChanRX, Gilead, Merck, Menarini, Otsuka, Sanofi, Servier, Xention, Bayer, Boehringer Ingelheim, Bristol- Myers Squibb, Daiichi Sankyo, Pfizer, Boston Scientific, Biotronik, Medtronic, St. Jude Medical, Actelion, GlaxoSmithKline, InfoBionic, Incarda, Johnson and Johnson, Mitsubishi, Novartis, Takeda Therapy for Ventricular Tachycardia Medical therapy Antiarrhythmic drugs Autonomic management Ventricular tachycardia Monomorphic Polymorphic Ventricular fibrillation Ventricular storms Ablation therapy Device therapy Surgical Defibrillation Catheter Antitachycardia pacing History of Antiarrhythmic Drugs 1914 - Quinidine 1950 - Lidocaine 1951 - Procainamide 1946 – Digitalis 1956 – Ajmaline 1962 - Verapamil 1962 – Disopyramide 1964 - Propranolol 1967 – Amiodarone 1965 – Bretylium 1972 – Mexiletine 1973 – Aprindine, Tocainide 1969 - Diltiazem 1975- Flecainide 1976 – Propafenone Encainide Ethmozine 2000 - Sotalol D-sotalol 1995 - Ibutilide (US) Recainam 2000 – Dofetilide US) IndecainideX Etc. -

Pharmaceutical Appendix to the Tariff Schedule 2
Harmonized Tariff Schedule of the United States (2006) – Supplement 1 (Rev. 1) Annotated for Statistical Reporting Purposes PHARMACEUTICAL APPENDIX TO THE HARMONIZED TARIFF SCHEDULE Harmonized Tariff Schedule of the United States (2006) – Supplement 1 (Rev. 1) Annotated for Statistical Reporting Purposes PHARMACEUTICAL APPENDIX TO THE TARIFF SCHEDULE 2 Table 1. This table enumerates products described by International Non-proprietary Names (INN) which shall be entered free of duty under general note 13 to the tariff schedule. The Chemical Abstracts Service (CAS) registry numbers also set forth in this table are included to assist in the identification of the products concerned. For purposes of the tariff schedule, any references to a product enumerated in this table includes such product by whatever name known. Product CAS No. Product CAS No. ABACAVIR 136470-78-5 ACEXAMIC ACID 57-08-9 ABAFUNGIN 129639-79-8 ACICLOVIR 59277-89-3 ABAMECTIN 65195-55-3 ACIFRAN 72420-38-3 ABANOQUIL 90402-40-7 ACIPIMOX 51037-30-0 ABARELIX 183552-38-7 ACITAZANOLAST 114607-46-4 ABCIXIMAB 143653-53-6 ACITEMATE 101197-99-3 ABECARNIL 111841-85-1 ACITRETIN 55079-83-9 ABIRATERONE 154229-19-3 ACIVICIN 42228-92-2 ABITESARTAN 137882-98-5 ACLANTATE 39633-62-0 ABLUKAST 96566-25-5 ACLARUBICIN 57576-44-0 ABUNIDAZOLE 91017-58-2 ACLATONIUM NAPADISILATE 55077-30-0 ACADESINE 2627-69-2 ACODAZOLE 79152-85-5 ACAMPROSATE 77337-76-9 ACONIAZIDE 13410-86-1 ACAPRAZINE 55485-20-6 ACOXATRINE 748-44-7 ACARBOSE 56180-94-0 ACREOZAST 123548-56-1 ACEBROCHOL 514-50-1 ACRIDOREX 47487-22-9 ACEBURIC -

Patent Application Publication ( 10 ) Pub . No . : US 2019 / 0192440 A1
US 20190192440A1 (19 ) United States (12 ) Patent Application Publication ( 10) Pub . No. : US 2019 /0192440 A1 LI (43 ) Pub . Date : Jun . 27 , 2019 ( 54 ) ORAL DRUG DOSAGE FORM COMPRISING Publication Classification DRUG IN THE FORM OF NANOPARTICLES (51 ) Int . CI. A61K 9 / 20 (2006 .01 ) ( 71 ) Applicant: Triastek , Inc. , Nanjing ( CN ) A61K 9 /00 ( 2006 . 01) A61K 31/ 192 ( 2006 .01 ) (72 ) Inventor : Xiaoling LI , Dublin , CA (US ) A61K 9 / 24 ( 2006 .01 ) ( 52 ) U . S . CI. ( 21 ) Appl. No. : 16 /289 ,499 CPC . .. .. A61K 9 /2031 (2013 . 01 ) ; A61K 9 /0065 ( 22 ) Filed : Feb . 28 , 2019 (2013 .01 ) ; A61K 9 / 209 ( 2013 .01 ) ; A61K 9 /2027 ( 2013 .01 ) ; A61K 31/ 192 ( 2013. 01 ) ; Related U . S . Application Data A61K 9 /2072 ( 2013 .01 ) (63 ) Continuation of application No. 16 /028 ,305 , filed on Jul. 5 , 2018 , now Pat . No . 10 , 258 ,575 , which is a (57 ) ABSTRACT continuation of application No . 15 / 173 ,596 , filed on The present disclosure provides a stable solid pharmaceuti Jun . 3 , 2016 . cal dosage form for oral administration . The dosage form (60 ) Provisional application No . 62 /313 ,092 , filed on Mar. includes a substrate that forms at least one compartment and 24 , 2016 , provisional application No . 62 / 296 , 087 , a drug content loaded into the compartment. The dosage filed on Feb . 17 , 2016 , provisional application No . form is so designed that the active pharmaceutical ingredient 62 / 170, 645 , filed on Jun . 3 , 2015 . of the drug content is released in a controlled manner. Patent Application Publication Jun . 27 , 2019 Sheet 1 of 20 US 2019 /0192440 A1 FIG . -

Safety and Efficacy of Vernakalant for the Conversion of Atrial Fibrillation to Sinus Rhythm; a Phase 3B Randomized Controlled Trial Gregory N
Beatch and Mangal BMC Cardiovascular Disorders (2016) 16:113 DOI 10.1186/s12872-016-0289-0 RESEARCH ARTICLE Open Access Safety and efficacy of vernakalant for the conversion of atrial fibrillation to sinus rhythm; a phase 3b randomized controlled trial Gregory N. Beatch1* and Brian Mangal2 Abstract Background: Atrial fibrillation (AF) is a common cardiac arrhythmia that is associated with significant health risks. One strategy to mitigate the risks associated with long-term AF is to convert AF to sinus rhythm (SR). This study assessed the efficacy and safety of vernakalant hydrochloride for the pharmacological conversion of AF to SR. Methods: Patients with recent-onset (duration >3 h– ≤ 7 days) symptomatic AF and no evidence or history of congestive heart failure were randomized in a 2:1 ratio to receive vernakalant or placebo. Patients received an infusion of vernakalant (3 mg/kg) or placebo over 10 min, followed by a second infusion of vernakalant (2 mg/kg) or placebo 15 min later if AF had not been terminated. The primary efficacy endpoint was conversion of AF to SR for at least 1 min within 90 min of the start of drug infusion. The primary safety endpoint was a composite of: occurrence of clinically significant hypotension, clinically significant ventricular arrhythmia (including torsades de pointes, ventricular tachycardia or ventricular fibrillation) or death within 2 h of starting the drug infusion. Results: A total of 217 patients were randomized to receive vernakalant (n = 145) or placebo (n =72).Ofthe129 individuals who received vernakalant, 59 (45.7 %) converted to SR compared with one of the 68 patients (1.5 %) who received placebo (p < 0.0001). -

MICROMEDEX® Healthcare Series
MICROMEDEX®Case 3:09-cv-00080-TMB Healthcare Series :Document Document 78-31 Filed 03/24/2010 Page 1 Paofg 209e 1 of 127 DRUGDEX® Evaluations RISPERIDONE 0.0 Overview 1) Class a) This drug is a member of the following class(es): Antipsychotic Benzisoxazole 2) Dosing Information a) Adult 1) if overlapping of antipsychotics is necessary, in all cases, the period of overlapping should be minimized; if swi antipsychotics and if medically appropriate, initiate risperidone therapy in place of the next scheduled injection (Pr RISPERDAL M-TAB(R) tablets, oral solution, orally disintegrating tablets, 2005) 2) previous oral antipsychotics should be continued for 3 weeks following the initiation of therapy with risperidone that adequate therapeutic concentrations are maintained until the main release phase of risperidone from the injec RISPERDAL(R) CONSTA(R) long acting injection, 2009) a) Bipolar I disorder 1) (oral, monotherapy or in combination with lithium or valproate) initial, 2 to 3 mg ORALLY once a day ( tablets, 2007; Prod Info RISPERDAL(R) M-TAB orally disintegrating tablets, 2007; Prod Info RISPERDA 2) (oral, monotherapy or in combination with lithium or valproate) maintenance, dosage adjustments sho mg/day at intervals of at least 24 hours; doses higher than 6 mg/day have not been evaluated in clinical t oral tablets, 2007; Prod Info RISPERDAL(R) M-TAB orally disintegrating tablets, 2007; Prod Info RISPER 3) (intramuscular, monotherapy or in combination with lithium or valproate) initiation of therapy, recomm oral risperidone prior to