Thematic Review
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Effects of Glycosylation on the Enzymatic Activity and Mechanisms of Proteases
International Journal of Molecular Sciences Review Effects of Glycosylation on the Enzymatic Activity and Mechanisms of Proteases Peter Goettig Structural Biology Group, Faculty of Molecular Biology, University of Salzburg, Billrothstrasse 11, 5020 Salzburg, Austria; [email protected]; Tel.: +43-662-8044-7283; Fax: +43-662-8044-7209 Academic Editor: Cheorl-Ho Kim Received: 30 July 2016; Accepted: 10 November 2016; Published: 25 November 2016 Abstract: Posttranslational modifications are an important feature of most proteases in higher organisms, such as the conversion of inactive zymogens into active proteases. To date, little information is available on the role of glycosylation and functional implications for secreted proteases. Besides a stabilizing effect and protection against proteolysis, several proteases show a significant influence of glycosylation on the catalytic activity. Glycans can alter the substrate recognition, the specificity and binding affinity, as well as the turnover rates. However, there is currently no known general pattern, since glycosylation can have both stimulating and inhibiting effects on activity. Thus, a comparative analysis of individual cases with sufficient enzyme kinetic and structural data is a first approach to describe mechanistic principles that govern the effects of glycosylation on the function of proteases. The understanding of glycan functions becomes highly significant in proteomic and glycomic studies, which demonstrated that cancer-associated proteases, such as kallikrein-related peptidase 3, exhibit strongly altered glycosylation patterns in pathological cases. Such findings can contribute to a variety of future biomedical applications. Keywords: secreted protease; sequon; N-glycosylation; O-glycosylation; core glycan; enzyme kinetics; substrate recognition; flexible loops; Michaelis constant; turnover number 1. -

University of California, San Diego
UNIVERSITY OF CALIFORNIA, SAN DIEGO A Lipidomic Perspective on Inflammatory Macrophage Eicosanoid Signaling A Thesis submitted in partial satisfaction of the requirements for the degree Doctor of Philosophy in Chemistry by Paul Christopher Norris Committee in charge: Professor Edward A. Dennis, Chair Professor Pieter C. Dorrestein Professor Partho Ghosh Professor Christopher K. Glass Professor Michael J. Sailor 2013 The Dissertation of Paul Christopher Norris is approved, and it is acceptable in quality and form for publication on microfilm and electronically: Chair University of California, San Diego 2013 iii DEDICATION To my parents, Darrell and Kathy, for always allowing me to think (and choose) for myself. iv TABLE OF CONTENTS Signature page ............................................................................................................................ iii Dedication .................................................................................................................................. iv Table of contents ......................................................................................................................... v List of symbols and abbreviations ........................................................................................... viii List of figures ............................................................................................................................. xi List of tables ............................................................................................................................ -

Montelukast, a Leukotriene Receptor Antagonist, Reduces the Concentration of Leukotrienes in the Respiratory Tract of Children with Persistent Asthma
Montelukast, a leukotriene receptor antagonist, reduces the concentration of leukotrienes in the respiratory tract of children with persistent asthma Benjamin Volovitz, MD,a,b Elvan Tabachnik, MD,c Moshe Nussinovitch, MD,b Biana Shtaif, MSc,b Hanna Blau, MD,a Irit Gil-Ad, PhD,b Abraham Weizman, MD,b and Itzhak Varsano, MDa,b Petah Tikva, Tel Aviv, and Rehovot, Israel Background: Leukotrienes are bronchoactive mediators secreted by inflammatory cells in the respiratory mucosa on Abbreviations used exposure to asthma triggers. BAL: Bronchoalveolar lavage Objective: We investigated the effect of montelukast, a CysLT1: Cysteinyl leukotriene 1 (receptor) leukotriene receptor antagonist, on the release of leukotrienes ECP: Eosinophilic cationic protein in the respiratory mucosa of children with persistent asthma. LTC4: Leukotriene C4 Method: Twenty-three children aged 6 to 11 years with moder- LTD4: Leukotriene D4 ately severe asthma were treated in a cross-over design start- LTE4: Leukotriene E4 ing, after a 2-week run in period, with either montelukast (n = 12) or cromolyn (n = 11) for 4 weeks with a 2-week washout period between treatments. Twelve of them were then treated Cysteinyl leukotrienes are potent proinflammatory with either montelukast or beclomethasone for 6 months. The mediators produced from a variety of inflammatory use of β -agonists was recorded on a diary card. The concen- 2 cells, including mast cells, eosinophils, basophils and tration of leukotriene C4 (LTC4) was measured by HPLC in nasal washes obtained before and at the end of each treatment macrophages. Leukotriene C4 (LTC4) is metabolized period. Eosinophilic cationic protein (ECP) was measured in enzymatically to leukotriene D4 (LTD4) and subsequent- the nasal washes by RIA. -

MALE Protein Name Accession Number Molecular Weight CP1 CP2 H1 H2 PDAC1 PDAC2 CP Mean H Mean PDAC Mean T-Test PDAC Vs. H T-Test
MALE t-test t-test Accession Molecular H PDAC PDAC vs. PDAC vs. Protein Name Number Weight CP1 CP2 H1 H2 PDAC1 PDAC2 CP Mean Mean Mean H CP PDAC/H PDAC/CP - 22 kDa protein IPI00219910 22 kDa 7 5 4 8 1 0 6 6 1 0.1126 0.0456 0.1 0.1 - Cold agglutinin FS-1 L-chain (Fragment) IPI00827773 12 kDa 32 39 34 26 53 57 36 30 55 0.0309 0.0388 1.8 1.5 - HRV Fab 027-VL (Fragment) IPI00827643 12 kDa 4 6 0 0 0 0 5 0 0 - 0.0574 - 0.0 - REV25-2 (Fragment) IPI00816794 15 kDa 8 12 5 7 8 9 10 6 8 0.2225 0.3844 1.3 0.8 A1BG Alpha-1B-glycoprotein precursor IPI00022895 54 kDa 115 109 106 112 111 100 112 109 105 0.6497 0.4138 1.0 0.9 A2M Alpha-2-macroglobulin precursor IPI00478003 163 kDa 62 63 86 72 14 18 63 79 16 0.0120 0.0019 0.2 0.3 ABCB1 Multidrug resistance protein 1 IPI00027481 141 kDa 41 46 23 26 52 64 43 25 58 0.0355 0.1660 2.4 1.3 ABHD14B Isoform 1 of Abhydrolase domain-containing proteinIPI00063827 14B 22 kDa 19 15 19 17 15 9 17 18 12 0.2502 0.3306 0.7 0.7 ABP1 Isoform 1 of Amiloride-sensitive amine oxidase [copper-containing]IPI00020982 precursor85 kDa 1 5 8 8 0 0 3 8 0 0.0001 0.2445 0.0 0.0 ACAN aggrecan isoform 2 precursor IPI00027377 250 kDa 38 30 17 28 34 24 34 22 29 0.4877 0.5109 1.3 0.8 ACE Isoform Somatic-1 of Angiotensin-converting enzyme, somaticIPI00437751 isoform precursor150 kDa 48 34 67 56 28 38 41 61 33 0.0600 0.4301 0.5 0.8 ACE2 Isoform 1 of Angiotensin-converting enzyme 2 precursorIPI00465187 92 kDa 11 16 20 30 4 5 13 25 5 0.0557 0.0847 0.2 0.4 ACO1 Cytoplasmic aconitate hydratase IPI00008485 98 kDa 2 2 0 0 0 0 2 0 0 - 0.0081 - 0.0 -

LEUKOTRIENE A4 HYDROLASE Martin J. Mueller
Department of Medical Biochemistry and Biophysics, Division of Chemistry II, Karolinska Institutet, 171 77 Stockholm, SWEDEN LEUKOTRIENE A4 HYDROLASE Identification of amino acid residues involved in catalyses and substrate-mediated inactivation Martin J. Mueller Stockholm 2001 Published and printed by Karolinska University Press Box 200, SE-171 77 Stockholm, Sweden © Martin J. Mueller, 2001 ISBN 91-628-4934-4 Abstract Leukotriene (LT) A4 hydrolase catalyzes the committed step in the biosynthesis of LTB4, a classical chemoattractant and immune-modulating lipid mediator involved in inflammation, host-defense against infections, and systemic, PAF-mediated, lethal shock. LTA4 hydrolase is a bifunctional zinc metalloenzyme with a chloride-stimulated arginyl aminopeptidase activity. When exposed to its lipid substrate LTA4, the enzyme is inactivated and covalently modified in a process termed suicide inactivation, which puts a restrain on the enzyme's ability to form the biologically active LTB4. In the present thesis, chemical modification with a series of amino acid-specific reagents, in the presence and absence of competitive inhibitors, was used to identify catalytically important residues at the active site. Thus, using differential labeling techniques, modification with the tyrosyl reagents N-acetylimidazole and tetranitromethane revealed the presence of two catalytically important Tyr residues. Likewise, modification with 2,3-butanedione and phenylglyoxal indicated that three Arg residues were located at, or near, the active center of the enzyme. Using differential Lys-specific peptide mapping of untreated and suicide inactivated LTA4 hy- drolase, a 21 residue peptide termed K21, was identified that is involved in binding of LTA4 to the native protein. Isolation and amino acid sequencing of a modified form of K21, revealed that Tyr- 378 is the site of attachment between LTA4 and the protein. -

Novel Proteins Regulated by Mtor in Subependymal Giant Cell Astrocytomas of Patients with Tuberous Sclerosis Complex and New Therapeutic Implications
The American Journal of Pathology, Vol. 176, No. 4, April 2010 Copyright © American Society for Investigative Pathology DOI: 10.2353/ajpath.2010.090950 Molecular Pathogenesis of Genetic and Inherited Diseases Novel Proteins Regulated by mTOR in Subependymal Giant Cell Astrocytomas of Patients with Tuberous Sclerosis Complex and New Therapeutic Implications Magdalena Ewa Tyburczy,* Katarzyna Kotulska,† and demonstrated an effective modulation of SEGA Piotr Pokarowski,‡ Jakub Mieczkowski,* growth by pharmacological inhibition of both Joanna Kucharska,* Wieslawa Grajkowska,† mTOR and extracellular signal-regulated kinase sig- Maciej Roszkowski,§ Sergiusz Jozwiak,† naling pathways, which could represent a novel ther- and Bozena Kaminska* apeutic approach. (Am J Pathol 2010, 176:1878–1890; DOI: 10.2353/ajpath.2010.090950) From the Laboratory of Transcription Regulation,* the Nencki Institute of Experimental Biology, Warsaw; the Departments of Neurology and Epileptology,† and Neurosurgery,§ the Children’s Subependymal giant cell astrocytomas (SEGAs) are rare, Memorial Health Institute, Warsaw; and the Faculty of low-grade brain tumors (World Health Organization Grade I) Mathematics, Informatics, and Mechanics,‡ University of of a mixed glioneuronal lineage.1,2 They are observed in Warsaw, Poland 10% to 20% of patients with tuberous sclerosis complex (TSC) and are the major cause of morbidity in children and young adults with TSC.3 The disease affects about one in Subependymal giant cell astrocytomas (SEGAs) are 6000 people, is characterized by the formation of benign rare brain tumors associated with tuberous sclerosis tumors in multiple organs (mainly brain, heart, kidneys, skin, complex (TSC), a disease caused by mutations in TSC1 or lungs), and is often associated with epilepsy, mental or TSC2, resulting in enhancement of mammalian retardation, and autism.4,5 Tuberous sclerosis complex is target of rapamycin (mTOR) activity, dysregulation of caused by mutation in one of two tumor suppressor genes, cell growth, and tumorigenesis. -

Unlocking the Non-Ige-Mediated Pseudo-Allergic Reaction Puzzle with Mas-Related G-Protein Coupled Receptor Member X2 (MRGPRX2)
cells Review Unlocking the Non-IgE-Mediated Pseudo-Allergic Reaction Puzzle with Mas-Related G-Protein Coupled Receptor Member X2 (MRGPRX2) Mukesh Kumar, Karthi Duraisamy and Billy-Kwok-Chong Chow * School of Biological Sciences, The University of Hong Kong, Pokfulam Road, Hong Kong, China; [email protected] (M.K.); [email protected] (K.D.) * Correspondence: [email protected]; Tel.: +852-2299-0850; Fax: +852-2559-9114 Abstract: Mas-related G-protein coupled receptor member X2 (MRGPRX2) is a class A GPCR ex- pressed on mast cells. Mast cells are granulated tissue-resident cells known for host cell response, allergic response, and vascular homeostasis. Immunoglobulin E receptor (Fc"RI)-mediated mast cell activation is a well-studied and recognized mechanism of allergy and hypersensitivity reac- tions. However, non-IgE-mediated mast cell activation is less explored and is not well recognized. After decades of uncertainty, MRGPRX2 was discovered as the receptor responsible for non-IgE- mediated mast cells activation. The puzzle of non-IgE-mediated pseudo-allergic reaction is unlocked by MRGPRX2, evidenced by a plethora of reported endogenous and exogenous MRGPRX2 ag- onists. MRGPRX2 is exclusively expressed on mast cells and exhibits varying affinity for many molecules such as antimicrobial host defense peptides, neuropeptides, and even US Food and Drug Administration-approved drugs. The discovery of MRGPRX2 has changed our understanding of mast cell biology and filled the missing link of the underlying mechanism of drug-induced MC degranulation and pseudo-allergic reactions. These non-canonical characteristics render MRGPRX2 Citation: Kumar, M.; Duraisamy, K.; Chow, B.-K.-C. -

Inflammation, Cancer and Oxidative Lipoxygenase Activity Are Intimately Linked
Cancers 2014, 6, 1500-1521; doi:10.3390/cancers6031500 OPEN ACCESS cancers ISSN 2072-6694 www.mdpi.com/journal/cancers Review Inflammation, Cancer and Oxidative Lipoxygenase Activity are Intimately Linked Rosalina Wisastra and Frank J. Dekker * Pharmaceutical Gene Modulation, Groningen Research Institute of Pharmacy, University of Groningen, Antonius Deusinglaan 1, 9713 AV Groningen, The Netherlands; E-Mail: [email protected] * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +31-5-3638030; Fax: +31-5-3637953. Received: 16 April 2014; in revised form: 27 June 2014 / Accepted: 2 July 2014 / Published: 17 July 2014 Abstract: Cancer and inflammation are intimately linked due to specific oxidative processes in the tumor microenvironment. Lipoxygenases are a versatile class of oxidative enzymes involved in arachidonic acid metabolism. An increasing number of arachidonic acid metabolites is being discovered and apart from their classically recognized pro-inflammatory effects, anti-inflammatory effects are also being described in recent years. Interestingly, these lipid mediators are involved in activation of pro-inflammatory signal transduction pathways such as the nuclear factor κB (NF-κB) pathway, which illustrates the intimate link between lipid signaling and transcription factor activation. The identification of the role of arachidonic acid metabolites in several inflammatory diseases led to a significant drug discovery effort around arachidonic acid metabolizing enzymes. However, to date success in this area has been limited. This might be attributed to the lack of selectivity of the developed inhibitors and to a lack of detailed understanding of the functional roles of arachidonic acid metabolites in inflammatory responses and cancer. -

Recombinant Human Dipeptidase 1 Protein
Leader in Biomolecular Solutions for Life Science Recombinant Human Dipeptidase 1 Protein Catalog No.: RP01097 Recombinant Sequence Information Background Species Gene ID Swiss Prot This protein is a kidney membrane enzyme involved in the metabolism of Human 1800 P16444 glutathione and other similar proteins by dipeptide hydrolysis. The encoded protein is known to regulate leukotriene activity by catalyzing the conversion of Tags leukotriene D4 to leukotriene E4. This protein uses zinc as a cofactor and acts as a C-6×His disulfide-linked homodimer. Two transcript variants encoding the same protein have been found for this gene. Synonyms Dipeptidase 1; Dehydropeptidase-I; Microsomal Dipeptidase; Renal Basic Information Dipeptidase; hRDP; DPEP1; MDP; RDP Description Recombinant Human Dipeptidase 1 Protein is produced by Mammalian expression Product Information system. The target protein is expressed with sequence (Asp17-Ser385) of human Dipeptidase 1 (Accession #P16444) fused with a 6×His tag at the C-terminus. Source Purification Mammalian > 95% by SDS- Bio-Activity PAGE. Storage Endotoxin Store the lyophilized protein at -20°C to -80 °C for long term. < 1.0 EU/μg of the protein by LAL After reconstitution, the protein solution is stable at -20 °C for 3 months, at 2-8 °C method. for up to 1 week. Avoid repeated freeze/thaw cycles. Formulation Lyophilized from a 0.22 μm filtered solution of 20mM PB, 150mM NaCl, pH7.4.Contact us for customized product form or formulation. Reconstitution Reconstitute to a concentration of 0.1-0.5 mg/mL in sterile distilled water. Contact www.abclonal.com Validation Data Recombinant Human Dipeptidase 1 Protein was determined by SDS-PAGE with Coomassie Blue, showing a band at 41 kDa. -
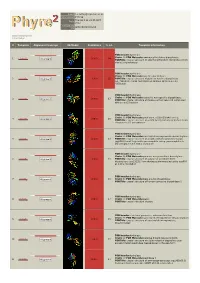
Phyre 2 Results for P15288
Email [email protected] Description P15288 Thu Jan 5 11:34:45 GMT Date 2012 Unique Job d2087d8d303c52c9 ID Detailed template information # Template Alignment Coverage 3D Model Confidence % i.d. Template Information PDB header:hydrolase Chain: B: PDB Molecule:aminoacyl-histidine dipeptidase; 1 c3mruB_ 100.0 64 Alignment PDBTitle: crystal structure of aminoacylhistidine dipeptidase from vibrio2 alginolyticus PDB header:hydrolase Chain: B: PDB Molecule:xaa-his dipeptidase; 2 c2qyvB_ Alignment 100.0 55 PDBTitle: crystal structure of putative xaa-his dipeptidase (yp_718209.1) from2 haemophilus somnus 129pt at 2.11 a resolution PDB header:hydrolase Chain: A: PDB Molecule:cytosolic non-specific dipeptidase; 3 c2zogA_ 100.0 17 Alignment PDBTitle: crystal structure of mouse carnosinase cn2 complexed with zn and2 bestatin PDB header:hydrolase Chain: B: PDB Molecule:peptidase, m20/m25/m40 family; 4 c2pokB_ 100.0 16 Alignment PDBTitle: crystal structure of a m20 family metallo peptidase from streptococcus2 pneumoniae PDB header:hydrolase Chain: A: PDB Molecule:succinyl-diaminopimelate desuccinylase; 5 c3pfeA_ Alignment 100.0 13 PDBTitle: crystal structure of a m20a metallo peptidase (dape, lpg0809) from2 legionella pneumophila subsp. pneumophila str. philadelphia 1 at 1.503 a resolution PDB header:hydrolase Chain: B: PDB Molecule:putative acetylornithine deacetylase; 6 c3pfoB_ Alignment 100.0 16 PDBTitle: crystal structure of a putative acetylornithine deacetylase (rpa2325)2 from rhodopseudomonas palustris cga009 at 1.90 a resolution PDB -

Levels of Prostaglandin E Metabolite And
Published OnlineFirst March 31, 2009; DOI: 10.1158/1940-6207.CAPR-09-0005 Published Online First on March 31, 2009 as 10.1158/1940-6207.CAPR-09-0005 Cancer Prevention Research Levels of Prostaglandin E Metabolite and Leukotriene E4 Are Increased in the Urine of Smokers: Evidence that Celecoxib Shunts Arachidonic Acid into the 5-Lipoxygenase Pathway Anna J. Duffield-Lillico,1,2 Jay O. Boyle,2 Xi Kathy Zhou,3 Aradhana Ghosh,4 Geera S. Butala,2 Kotha Subbaramaiah,4 Robert A. Newman,5 Jason D. Morrow,6 Ginger L. Milne6 and Andrew J. Dannenberg4 Abstract Cyclooxygenase-2 (COX-2) and 5-lipoxygenase (5-LO) play a role in inflammation and car- cinogenesis. Biomarkers that reflect tobacco smoke–induced tissue injury are needed. In this study, levels of urinary prostaglandin E metabolite (PGE-M) and leukotriene E4 (LTE4), biomarkers of the COX and 5-LO pathways, were compared in never smokers, former smo- kers, and current smokers. The effects of celecoxib, a selective COX-2 inhibitor, on levels of PGE-M and LTE4 were determined. Baseline levels of PGE-M and LTE4 were positively as- sociated with smoking status; levels of PGE-M and LTE4 were higher in current versus never smokers. Treatment with 200 mg celecoxib twice daily for 6 ± 1 days led to a reduction in urinary PGE-M levels in all groups but exhibited the greatest effect among subjects with high baseline PGE-M levels. Thus, high baseline PGE-M levels in smokers reflected increased COX-2 activity. In individuals with high baseline PGE-M levels, treatment with celecoxib led to a significant increase in levels of urinary LTE4, an effect that was not found in indivi- duals with low baseline PGE-M levels. -

Signaling and Regulation of Cysteinyl Leukotriene Receptors in Intestinal Epithelial Cells and Colon Cancer Bengtsson, Astrid
Signaling and regulation of cysteinyl leukotriene receptors in intestinal epithelial cells and colon cancer Bengtsson, Astrid 2009 Link to publication Citation for published version (APA): Bengtsson, A. (2009). Signaling and regulation of cysteinyl leukotriene receptors in intestinal epithelial cells and colon cancer. Lund University. Total number of authors: 1 General rights Unless other specific re-use rights are stated the following general rights apply: Copyright and moral rights for the publications made accessible in the public portal are retained by the authors and/or other copyright owners and it is a condition of accessing publications that users recognise and abide by the legal requirements associated with these rights. • Users may download and print one copy of any publication from the public portal for the purpose of private study or research. • You may not further distribute the material or use it for any profit-making activity or commercial gain • You may freely distribute the URL identifying the publication in the public portal Read more about Creative commons licenses: https://creativecommons.org/licenses/ Take down policy If you believe that this document breaches copyright please contact us providing details, and we will remove access to the work immediately and investigate your claim. LUND UNIVERSITY PO Box 117 221 00 Lund +46 46-222 00 00 From the Department of Laboratory Medicine, Division of Cell Pathology, Lund University, Malmö, Sweden Signaling and regulation of cysteinyl leukotriene receptors in intestinal epithelial cells and colon cancer Astrid Bengtsson Academic dissertation By due permission of the Faculty of Medicine, Lund University, Sweden, to be publicly defended in the lecture hall, Clinical Research Center, Entrance 72, Malmö University Hospital (UMAS), Malmö on Friday, May 15, 2009, at 1 p.m.