Fermi-Dirac Distribution
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Fermi- Dirac Statistics
Prof. Surajit Dhara Guest Teacher, Dept. Of Physics, Narajole Raj College GE2T (Thermal physics and Statistical Mechanics) , Topic :- Fermi- Dirac Statistics Fermi- Dirac Statistics Basic features: The basic features of the Fermi-Dirac statistics: 1. The particles are all identical and hence indistinguishable. 2. The fermions obey (a) the Heisenberg’s uncertainty relation and also (b) the exclusion principle of Pauli. 3. As a consequence of 2(a), there exists a number of quantum states for a given energy level and because of 2(b) , there is a definite a priori restriction on the number of fermions in a quantum state; there can be simultaneously no more than one particle in a quantum state which would either remain empty or can at best contain one fermion. If , again, the particles are isolated and non-interacting, the following two additional condition equations apply to the system: ∑ ; Thermodynamic probability: Consider an isolated system of N indistinguishable, non-interacting particles obeying Pauli’s exclusion principle. Let particles in the system have energies respectively and let denote the degeneracy . So the number of distinguishable arrangements of particles among eigenstates in the ith energy level is …..(1) Therefore, the thermodynamic probability W, that is, the total number of s eigenstates of the whole system is the product of ……(2) GE2T (Thermal physics and Statistical Mechanics), Topic :- Fermi- Dirac Statistics: Circulated by-Prof. Surajit Dhara, Dept. Of Physics, Narajole Raj College FD- distribution function : Most probable distribution : From eqn(2) above , taking logarithm and applying Stirling’s theorem, For this distribution to represent the most probable distribution , the entropy S or klnW must be maximum, i.e. -
Lecture 24. Degenerate Fermi Gas (Ch
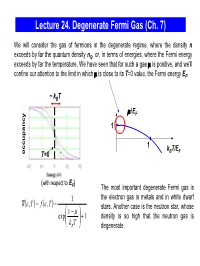
Lecture 24. Degenerate Fermi Gas (Ch. 7) We will consider the gas of fermions in the degenerate regime, where the density n exceeds by far the quantum density nQ, or, in terms of energies, where the Fermi energy exceeds by far the temperature. We have seen that for such a gas μ is positive, and we’ll confine our attention to the limit in which μ is close to its T=0 value, the Fermi energy EF. ~ kBT μ/EF 1 1 kBT/EF occupancy T=0 (with respect to E ) F The most important degenerate Fermi gas is 1 the electron gas in metals and in white dwarf nε()(),, T= f ε T = stars. Another case is the neutron star, whose ε⎛ − μ⎞ exp⎜ ⎟ +1 density is so high that the neutron gas is ⎝kB T⎠ degenerate. Degenerate Fermi Gas in Metals empty states ε We consider the mobile electrons in the conduction EF conduction band which can participate in the charge transport. The band energy is measured from the bottom of the conduction 0 band. When the metal atoms are brought together, valence their outer electrons break away and can move freely band through the solid. In good metals with the concentration ~ 1 electron/ion, the density of electrons in the electron states electron states conduction band n ~ 1 electron per (0.2 nm)3 ~ 1029 in an isolated in metal electrons/m3 . atom The electrons are prevented from escaping from the metal by the net Coulomb attraction to the positive ions; the energy required for an electron to escape (the work function) is typically a few eV. -

Chapter 13 Ideal Fermi
Chapter 13 Ideal Fermi gas The properties of an ideal Fermi gas are strongly determined by the Pauli principle. We shall consider the limit: k T µ,βµ 1, B � � which defines the degenerate Fermi gas. In this limit, the quantum mechanical nature of the system becomes especially important, and the system has little to do with the classical ideal gas. Since this chapter is devoted to fermions, we shall omit in the following the subscript ( ) that we used for the fermionic statistical quantities in the previous chapter. − 13.1 Equation of state Consider a gas ofN non-interacting fermions, e.g., electrons, whose one-particle wave- functionsϕ r(�r) are plane-waves. In this case, a complete set of quantum numbersr is given, for instance, by the three cartesian components of the wave vector �k and thez spin projectionm s of an electron: r (k , k , k , m ). ≡ x y z s Spin-independent Hamiltonians. We will consider only spin independent Hamiltonian operator of the type ˆ 3 H= �k ck† ck + d r V(r)c r†cr , �k � where thefirst and the second terms are respectively the kinetic and th potential energy. The summation over the statesr (whenever it has to be performed) can then be reduced to the summation over states with different wavevectork(p=¯hk): ... (2s + 1) ..., ⇒ r � �k where the summation over the spin quantum numberm s = s, s+1, . , s has been taken into account by the prefactor (2s + 1). − − 159 160 CHAPTER 13. IDEAL FERMI GAS Wavefunctions in a box. We as- sume that the electrons are in a vol- ume defined by a cube with sidesL x, Ly,L z and volumeV=L xLyLz. -

Recitation 8 Notes
3.024 Electrical, Optical, and Magnetic Properties of Materials Spring 2012 Recitation 8 Notes Overview 1. Electronic Band Diagram Review 2. Spin Review 3. Density of States 4. Fermi-Dirac Distribution 1. Electronic Band Diagram Review Considering 1D crystals with periodic potentials of the form: ( ) ∑ n a Here where is an integer, is the crystal lattice constant, and is the reciprocal space lattice constant. The Schrodinger equation for these systems has a general solution of the form: ( ) ∑ Upon substitution of this solution into Schrodinger’s equation, the following Central Equation is found: ( ) ∑ This is an eigenvalue/eigenvector equation where the eigenvalues are given by E and the eigenvectors by the coefficients Ck. By rewriting the above expression as a matrix equation, one can computationally solve for the energy eigenvalues for given values of k and produce a band diagram. ( ) ( ) ( ) ( ) [ ] [ ] The steps to solving this equation for just the energy eigenvalues is as follows: 1. Set values of equal to values of coefficients of the periodic potential of interest. 2. Truncate the infinite matrix to an matrix approximation suitable for the number of energy bands that is required for the problem to be investigated. If N is odd, set the center term to the Ck matrix coefficient. If N is even, an asymmetry will be introduced in the top band depending on whether the Ck matrix coefficient is placed to the left diagonal of the matrix center or the right diagonal of the matrix center. This asymmetry can be avoided by only using odd matrices or by solving both even matrices and superimposing the highest band energy solutions. -

The Physics of Electron Degenerate Matter in White Dwarf Stars
Western Michigan University ScholarWorks at WMU Master's Theses Graduate College 6-2008 The Physics of Electron Degenerate Matter in White Dwarf Stars Subramanian Vilayur Ganapathy Follow this and additional works at: https://scholarworks.wmich.edu/masters_theses Part of the Physics Commons Recommended Citation Ganapathy, Subramanian Vilayur, "The Physics of Electron Degenerate Matter in White Dwarf Stars" (2008). Master's Theses. 4251. https://scholarworks.wmich.edu/masters_theses/4251 This Masters Thesis-Open Access is brought to you for free and open access by the Graduate College at ScholarWorks at WMU. It has been accepted for inclusion in Master's Theses by an authorized administrator of ScholarWorks at WMU. For more information, please contact [email protected]. THE PHYSICS OF ELECTRON DEGENERATE MATTER IN WHITE DWARF STARS by Subramanian Vilayur Ganapathy A Thesis Submitted to the Faculty of The Graduate College in partial fulfillment of the requirements forthe Degree of Master of Arts Department of Physics Western MichiganUniversity Kalamazoo, Michigan June 2008 Copyright by Subramanian Vilayur Ganapathy 2008 ACKNOWLEDGMENTS I wish to express my infinite gratitude and sincere appreciation to my advisor, Professor Kirk Korista, for suggesting the topic of the thesis, and for all of his direction, encouragement and great help throughout the course of this research. I would also like to express my gratitude to the other members of my committee, Professor Dean Halderson and Professor Clement Bums for their valuable advice and help. Subramanian Vilayur Ganapathy 11 THE PHYSICS OF ELECTRON DEGENERATE MATTER IN WHITE DWARF STARS Subramanian Vilayur Ganapathy , M.A. Western Michigan University, 2008 White dwarfs are the remnant cores of medium and low mass stars with initial mass less than 8 times the mass of our sun. -

13 Classical and Quantum Statistics
James Maxwell (1831–1879) Ludwig Boltzmann (1844–1906) Enrico Fermi (1901–1954) Established velocity Established classical statistics Established quantum statistics distribution of gases 13 Classical and quantum statistics Classical Maxwell–Boltzmann statistics and quantum mechanical Fermi–Dirac statistics are introduced to calculate the occupancy of states. Special attention is given to analytic approximations of the Fermi–Dirac integral and to its approximate solutions in the non- degenerate and the highly degenerate regime. In addition, some numerical approximations to the Fermi–Dirac integral are summarized. Semiconductor statistics includes both classical statistics and quantum statistics. Classical or Maxwell–Boltzmann statistics is derived on the basis of purely classical physics arguments. In contrast, quantum statistics takes into account two results of quantum mechanics, namely (i) the Pauli exclusion principle which limits the number of electrons occupying a state of energy E and (ii) the finiteness of the number of states in an energy interval E and E + dE. The finiteness of states is a result of the Schrödinger equation. In this section, the basic concepts of classical statistics and quantum statistics are derived. The fundamentals of ideal gases and statistical distributions are summarized as well since they are the basis of semiconductor statistics. 13.1 Probability and distribution functions Consider a large number N of free classical particles such as atoms, molecules or electrons which are kept at a constant temperature T, and which interact only weakly with one another. The energy of a single particle consists of kinetic energy due to translatory motion and an internal energy for example due to rotations, vibrations, or orbital motions of the particle. -

§6 – Free Electron Gas : §6 – Free Electron Gas
§6 – Free Electron Gas : §6 – Free Electron Gas 6.1 Overview of Electronic Properties The main species of solids are metals, semiconductors and insulators. One way to group these is by their resistivity: Metals – ~10−8−10−5 m Semiconductors – ~10−5−10 m Insulators – ~10−∞ m There is a striking difference in the temperature dependence of the resistivity: Metals – ρ increases with T (usually ∝T ) Semiconductors – ρ decreases with T Alloys have higher resistivity than its constituents; there is no law of mixing. This suggests some form of “impurity” mechanism The different species also have different optical properties. Metals – Opaque and lustrous (silvery) Insulators – often transparent or coloured The reasons for these differing properties can be explained by considering the density of electron states: 6.2 Free Electron (Fermi) Gas The simplest model of a metal was proposed by Fermi. This model transfers the ideas of electron orbitals in atoms into a macroscopic object. Thus, the fundamental behaviour of a metal comes from the Pauli exclusion principle. For now, we will ignore the crystal lattice. – 1 – Last Modified: 28/12/2006 §6 – Free Electron Gas : For this model, we make the following assumptions: ● The crystal comprises a fixed background of N identical positively charge nuclei and N electrons, which can move freely inside the crystal without seeing any of the nuclei (monovalent case); ● Coulomb interactions are negligible because the system is neutral overall This model works relatively well for alkali metals (Group 1 elements), such as Na, K, Rb and Cs. We would like to understand why electrons are only weakly scattered as they migrate through a metal. -

Nuclear Models: the Liquid Drop Model Fermi-Gas Model
LectureLecture 22 NuclearNuclear models:models: TheThe liquidliquid dropdrop modelmodel FermiFermi --GasGas ModelModel WS2012/13 : ‚Introduction to Nuclear and Particle Physics ‘, Part I 1 NuclearNuclear modelsmodels Nuclear models Models with strong interaction between Models of non-interacting the nucleons nucleons Liquid drop model Fermi gas model ααα-particle model Optical model Shell model … … Nucleons interact with the nearest Nucleons move freely inside the nucleus: neighbors and practically don‘t move: mean free path λ ~ R A nuclear radius mean free path λ << R A nuclear radius 2 I.I. TheThe liquidliquid dropdrop modelmodel 3 TheThe liquidliquid dropdrop modelmodel The liquid drop model is a model in nuclear physics which treats the nucleus as a drop of incompressible nuclear fluid first proposed by George Gamow and developed by Niels Bohr and John Archibald Wheeler The fluid is made of nucleons (protons and neutrons), which are held together by the strong nuclear force . This is a crude model that does not explain all the properties of the nucleus, but (!) • does explain the spherical shape of most nuclei. • It also helps to predict the binding energy of the nucleus . George Gamow Niels Henrik David Bohr John Archibald Wheeler (1930-1968) (1885-1962) (1911-2008) 4 TheThe liquidliquid dropdrop modelmodel The parametrisation of nuclear masses as a function of A and Z, which is known as the Weizsäcker formula or the semi-empirical mass formula , was first introduced in 1935 by German physicist Carl Friedrich von Weizsäcker: M ( A, Z ) === Zm p +++ Nm n −−− E B EB is the binding energy of the nucleus : Volum term Surface term Coulomb term Assymetry term Pairing term Empirical parameters: 5 Binding energy of the nucleus Volume energy (dominant term): E V === aV A A - mass number Coefficient aV ≈≈≈ 16 MeV •The basis for this term is the strong nuclear force . -

LECTURE 13 Maxwell–Boltzmann, Fermi, and Bose Statistics Suppose We Have a Gas of N Identical Point Particles in a Box of Volume V
LECTURE 13 Maxwell–Boltzmann, Fermi, and Bose Statistics Suppose we have a gas of N identical point particles in a box of volume V. When we say “gas”, we mean that the particles are not interacting with one another. Suppose we know the single particle states in this gas. We would like to know what are the possible states of the system as a whole. There are 3 possible cases. Which one is appropriate depends on whether we use Maxwell–Boltzmann, Fermi or Bose statistics. Let’s consider a very simple case in which we have 2 particles in the box and the box has 2 single particle states. How many distinct ways can we put the particles into the 2 states? Maxwell–Boltzmann Statistics: This is sometimes called the classical case. In this case the particles are distinguishable so let’s label them A and B. Let’s call the 2 single particle states 1 and 2. For Maxwell–Boltzmann statistics any number of particles can be in any state. So let’s enumerate the states of the system: SingleParticleState 1 2 ----------------------------------------------------------------------- AB AB A B B A We get a total of 4 states of the system as a whole. Half of the states have the particles bunched in the same state and half have them in separate states. Bose–Einstein Statistics: This is a quantum mechanical case. This means that the particles are indistinguishable. Both particles are labelled A. Recall that bosons have integer spin: 0, 1, 2, etc. For Bose statistics any number of particles can be in one state. -

Lecture 15: Free Electron Gas up to This Point We Have Not Discussed
Lecture 15: free electron gas Up to this point we have not discussed electrons explicitly, but electrons are the first offenders for most useful phenomena in materials. When we distinguish between metals, insulators, semiconductors, and semimetals, we are discussing conduction properties of the electrons. When we notice that different materials have different colors, this is also usually due to electrons. Interactions with the lattice will influence electron behavior (which is why we studied them independently first), but electrons are the primary factor in materials’ response to electromagnetic stimulus (light or a potential difference), and this is how we usually use materials in modern technology. Electrons as particles A classical treatment of electrons in a solid, called the Drude model, considers valence electrons to act like billiard balls that scatter off each other and off lattice imperfections (including thermal vibrations). This model introduces important terminology and formalism that is still used to this day to describe materials’ response to electromagnetic radiation, but it is not a good physical model for electrons in most materials, so we will not discuss it in detail. Electrons as waves In chapter 3, when we discussed metallic bonding, the primary attribute was that electrons are delocalized. In quantum mechanical language, when something is delocalized, it means that its position is ill defined which means that its momentum is more well defined. An object with a well defined momentum but an ill-defined position is a plane-wave, and in this chapter we will treat electrons like plane waves, defined by their momentum. Another important constraint at this point is that electrons do not interact with each other, except for pauli exclusion (that is, two electrons cannot be in the same state, where a state is defined by a momentum and a spin). -

Degeneracy Pressure from Chapter 10, for Particles in a Box with Number Density N, Speed V, Momentum P, the Pressure Is
Astronomy 421 Lecture 22: End states of stars - White Dwarfs 1 Homework #7 – Astro 421 Review Panel You should have five (5) papers to read You have two (2) papers to provide a short written review You must rank all five papers and submit your ranking by Nov 11 We will discuss the papers in class on Tuesday, Nov 12 The class discussion counts 50% of the HW grade 2 Outline – White Dwarf Stars Sirius B White Dwarf Properties Electron degeneracy condition, pressure, relativistic pressure Mass-volume relation Chandrasekhar limit White dwarf cooling and evolution 3 Discovery of Sirius B aka “the pup” Trajectory of Sirius A is not a straight line: 4 Discovery of Sirius B aka “the pup” 5 Spectrum of Sirius B and A 6 X-ray image of Sirius B aka “the pup” 7 Discovery of Sirius B aka “the pup” 8 End states of stars Possibilities: 1. Violent explosion, no remnant (Type Ia SN) 2. White dwarf • M ~ 0.6M • R ~ R⊕ • ρ ~ 109 kg m-3 3. Neutron star • M ~ 1.4M • R ~ 10 km • ρ ~ 1017 kg m-3 4. Black hole • M ≥ 3M 9 White dwarfs – Most famous one is Sirius B – Visual binary, yields mass M ~ 1.05 M – From distance and spectrum, we get L, Teff. 3 4 This implies R ~ 5,500 km < R⊕ (range 10 - 10 km for WDs) – Teff = 27,000 K (range 5,000-80,000 K for WDs) 7 – central temperature Tc = several x 10 K – Should be isothermal except for very outer layers – efficient energy transport by electron conduction equalizes T. -

The Equation of State for Neutron Stars Laura Tolós Outline Neutron Star (I)
The Equation of State for Neutron Stars Laura Tolós Outline Neutron Star (I) • first observations by the Chinese in 1054 A.D. and prediction by Landau after discovery of neutron by Chadwick in 1932 • produced in core collapse supernova explosions • usually refer to compact objects with M≈1-2 M¤ and R≈10-12 Km • extreme densities up to 5-10 times nuclear density ρ0 -3 14 3 (ρ0=0.16 fm => n0=310 g/cm ) -30 3 nUniverse ~ 10 g/cm 3 nSun ~ 1.4 g/cm 3 nEarth ~ 5.5 g/cm Neutron Star (II) • usually observed as pulsars with rotational periods from milliseconds to seconds • magnetic field : B ~ 10 8..16 G • temperature: T ~ 10 6…11 K (1 eV=104 K) Pulsars are magnetized rotating neutron stars emitting a highly focused beam of electromagnetic radiation oriented long the magnetic axis. The misalignment between the magnetic axis and the spin axis leads to a lighthouse effect: from Earth we see pulses Since 1967, ∼ 2500 pulsars have been discovered. http://www.atnf.csiro.au/research/pulsar/psrcat/ Most of them have been detected as radio pulsars, but also some observed in X-rays Their period P ranges from and an increasingly large number detected in 1.396 ms for PSRJ1748−2446ad gamma rays. up to 8.5 s for PSR J2144−3933. Magnetic fields Bulk properties: Masses • > 2000 pulsars known • best determined masses: Hulse-Taylor pulsar M=1.4414 ± 0.0002 M¤ Hulse-Taylor Nobel Prize 94 figures by Weiseberg • PSR J1614-22301 M=(1.97 ± 0.04) M¤; PSR J0348+04322 M=(2.01 ± 0.04) M¤ 1Demorest et al ’10; 2Antoniadis et al ‘13 Lattimer ‘16 Bulk properties: Fortin et