§6 – Free Electron Gas : §6 – Free Electron Gas
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Part 5: the Bose-Einstein Distribution
PHYS393 – Statistical Physics Part 5: The Bose-Einstein Distribution Distinguishable and indistinguishable particles In the previous parts of this course, we derived the Boltzmann distribution, which described how the number of distinguishable particles in different energy states varied with the energy of those states, at different temperatures: N εj nj = e− kT . (1) Z However, in systems consisting of collections of identical fermions or identical bosons, the wave function of the system has to be either antisymmetric (for fermions) or symmetric (for bosons) under interchange of any two particles. With the allowed wave functions, it is no longer possible to identify a particular particle with a particular energy state. Instead, all the particles are “shared” between the occupied states. The particles are said to be indistinguishable . Statistical Physics 1 Part 5: The Bose-Einstein Distribution Indistinguishable fermions In the case of indistinguishable fermions, the wave function for the overall system must be antisymmetric under the interchange of any two particles. One consequence of this is the Pauli exclusion principle: any given state can be occupied by at most one particle (though we can’t say which particle!) For example, for a system of two fermions, a possible wave function might be: 1 ψ(x1, x 2) = [ψ (x1)ψ (x2) ψ (x2)ψ (x1)] . (2) √2 A B − A B Here, x1 and x2 are the coordinates of the two particles, and A and B are the two occupied states. If we try to put the two particles into the same state, then the wave function vanishes. Statistical Physics 2 Part 5: The Bose-Einstein Distribution Indistinguishable fermions Finding the distribution with the maximum number of microstates for a system of identical fermions leads to the Fermi-Dirac distribution: g n = i . -

Fermi- Dirac Statistics
Prof. Surajit Dhara Guest Teacher, Dept. Of Physics, Narajole Raj College GE2T (Thermal physics and Statistical Mechanics) , Topic :- Fermi- Dirac Statistics Fermi- Dirac Statistics Basic features: The basic features of the Fermi-Dirac statistics: 1. The particles are all identical and hence indistinguishable. 2. The fermions obey (a) the Heisenberg’s uncertainty relation and also (b) the exclusion principle of Pauli. 3. As a consequence of 2(a), there exists a number of quantum states for a given energy level and because of 2(b) , there is a definite a priori restriction on the number of fermions in a quantum state; there can be simultaneously no more than one particle in a quantum state which would either remain empty or can at best contain one fermion. If , again, the particles are isolated and non-interacting, the following two additional condition equations apply to the system: ∑ ; Thermodynamic probability: Consider an isolated system of N indistinguishable, non-interacting particles obeying Pauli’s exclusion principle. Let particles in the system have energies respectively and let denote the degeneracy . So the number of distinguishable arrangements of particles among eigenstates in the ith energy level is …..(1) Therefore, the thermodynamic probability W, that is, the total number of s eigenstates of the whole system is the product of ……(2) GE2T (Thermal physics and Statistical Mechanics), Topic :- Fermi- Dirac Statistics: Circulated by-Prof. Surajit Dhara, Dept. Of Physics, Narajole Raj College FD- distribution function : Most probable distribution : From eqn(2) above , taking logarithm and applying Stirling’s theorem, For this distribution to represent the most probable distribution , the entropy S or klnW must be maximum, i.e. -

The Debye Model of Lattice Heat Capacity
The Debye Model of Lattice Heat Capacity The Debye model of lattice heat capacity is more involved than the relatively simple Einstein model, but it does keep the same basic idea: the internal energy depends on the energy per phonon (ε=ℏΩ) times the ℏΩ/kT average number of phonons (Planck distribution: navg=1/[e -1]) times the number of modes. It is in the number of modes that the difference between the two models occurs. In the Einstein model, we simply assumed that each mode was the same (same frequency, Ω), and that the number of modes was equal to the number of atoms in the lattice. In the Debye model, we assume that each mode has its own K (that is, has its own λ). Since Ω is related to K (by the dispersion relation), each mode has a different Ω. 1. Number of modes In the Debye model we assume that the normal modes consist of STANDING WAVES. If we have travelling waves, they will carry energy through the material; if they contain the heat energy (that will not move) they need to be standing waves. Standing waves are waves that do travel but go back and forth and interfere with each other to create standing waves. Recall that Newton's Second Law gave us atoms that oscillate: [here x = sa where s is an integer and a the lattice spacing] iΩt iKsa us = uo e e ; and if we use the form sin(Ksa) for eiKsa [both indicate oscillations]: iΩt us = uo e sin(Ksa) . We now apply the boundary conditions on our solution: to have standing waves both ends of the wave must be tied down, that is, we must have u0 = 0 and uN = 0. -

Competition Between Paramagnetism and Diamagnetism in Charged
Competition between paramagnetism and diamagnetism in charged Fermi gases Xiaoling Jian, Jihong Qin, and Qiang Gu∗ Department of Physics, University of Science and Technology Beijing, Beijing 100083, China (Dated: November 10, 2018) The charged Fermi gas with a small Lande-factor g is expected to be diamagnetic, while that with a larger g could be paramagnetic. We calculate the critical value of the g-factor which separates the dia- and para-magnetic regions. In the weak-field limit, gc has the same value both at high and low temperatures, gc = 1/√12. Nevertheless, gc increases with the temperature reducing in finite magnetic fields. We also compare the gc value of Fermi gases with those of Boltzmann and Bose gases, supposing the particle has three Zeeman levels σ = 1, 0, and find that gc of Bose and Fermi gases is larger and smaller than that of Boltzmann gases,± respectively. PACS numbers: 05.30.Fk, 51.60.+a, 75.10.Lp, 75.20.-g I. INTRODUCTION mK and 10 µK, respectively. The ions can be regarded as charged Bose or Fermi gases. Once the quantum gas Magnetism of electron gases has been considerably has both the spin and charge degrees of freedom, there studied in condensed matter physics. In magnetic field, arises the competition between paramagnetism and dia- the magnetization of a free electron gas consists of two magnetism, as in electrons. Different from electrons, the independent parts. The spin magnetic moment of elec- g-factor for different magnetic ions is diverse, ranging trons results in the paramagnetic part (the Pauli para- from 0 to 2. -

Quantum Mechanics Electromotive Force
Quantum Mechanics_Electromotive force . Electromotive force, also called emf[1] (denoted and measured in volts), is the voltage developed by any source of electrical energy such as a batteryor dynamo.[2] The word "force" in this case is not used to mean mechanical force, measured in newtons, but a potential, or energy per unit of charge, measured involts. In electromagnetic induction, emf can be defined around a closed loop as the electromagnetic workthat would be transferred to a unit of charge if it travels once around that loop.[3] (While the charge travels around the loop, it can simultaneously lose the energy via resistance into thermal energy.) For a time-varying magnetic flux impinging a loop, theElectric potential scalar field is not defined due to circulating electric vector field, but nevertheless an emf does work that can be measured as a virtual electric potential around that loop.[4] In a two-terminal device (such as an electrochemical cell or electromagnetic generator), the emf can be measured as the open-circuit potential difference across the two terminals. The potential difference thus created drives current flow if an external circuit is attached to the source of emf. When current flows, however, the potential difference across the terminals is no longer equal to the emf, but will be smaller because of the voltage drop within the device due to its internal resistance. Devices that can provide emf includeelectrochemical cells, thermoelectric devices, solar cells and photodiodes, electrical generators,transformers, and even Van de Graaff generators.[4][5] In nature, emf is generated whenever magnetic field fluctuations occur through a surface. -

Phys 446: Solid State Physics / Optical Properties Lattice Vibrations
Solid State Physics Lecture 5 Last week: Phys 446: (Ch. 3) • Phonons Solid State Physics / Optical Properties • Today: Einstein and Debye models for thermal capacity Lattice vibrations: Thermal conductivity Thermal, acoustic, and optical properties HW2 discussion Fall 2007 Lecture 5 Andrei Sirenko, NJIT 1 2 Material to be included in the test •Factors affecting the diffraction amplitude: Oct. 12th 2007 Atomic scattering factor (form factor): f = n(r)ei∆k⋅rl d 3r reflects distribution of electronic cloud. a ∫ r • Crystalline structures. 0 sin()∆k ⋅r In case of spherical distribution f = 4πr 2n(r) dr 7 crystal systems and 14 Bravais lattices a ∫ n 0 ∆k ⋅r • Crystallographic directions dhkl = 2 2 2 1 2 ⎛ h k l ⎞ 2πi(hu j +kv j +lw j ) and Miller indices ⎜ + + ⎟ •Structure factor F = f e ⎜ a2 b2 c2 ⎟ ∑ aj ⎝ ⎠ j • Definition of reciprocal lattice vectors: •Elastic stiffness and compliance. Strain and stress: definitions and relation between them in a linear regime (Hooke's law): σ ij = ∑Cijklε kl ε ij = ∑ Sijklσ kl • What is Brillouin zone kl kl 2 2 C •Elastic wave equation: ∂ u C ∂ u eff • Bragg formula: 2d·sinθ = mλ ; ∆k = G = eff x sound velocity v = ∂t 2 ρ ∂x2 ρ 3 4 • Lattice vibrations: acoustic and optical branches Summary of the Last Lecture In three-dimensional lattice with s atoms per unit cell there are Elastic properties – crystal is considered as continuous anisotropic 3s phonon branches: 3 acoustic, 3s - 3 optical medium • Phonon - the quantum of lattice vibration. Elastic stiffness and compliance tensors relate the strain and the Energy ħω; momentum ħq stress in a linear region (small displacements, harmonic potential) • Concept of the phonon density of states Hooke's law: σ ij = ∑Cijklε kl ε ij = ∑ Sijklσ kl • Einstein and Debye models for lattice heat capacity. -

Solid State Physics 2 Lecture 5: Electron Liquid
Physics 7450: Solid State Physics 2 Lecture 5: Electron liquid Leo Radzihovsky (Dated: 10 March, 2015) Abstract In these lectures, we will study itinerate electron liquid, namely metals. We will begin by re- viewing properties of noninteracting electron gas, developing its Greens functions, analyzing its thermodynamics, Pauli paramagnetism and Landau diamagnetism. We will recall how its thermo- dynamics is qualitatively distinct from that of a Boltzmann and Bose gases. As emphasized by Sommerfeld (1928), these qualitative di↵erence are due to the Pauli principle of electons’ fermionic statistics. We will then include e↵ects of Coulomb interaction, treating it in Hartree and Hartree- Fock approximation, computing the ground state energy and screening. We will then study itinerate Stoner ferromagnetism as well as various response functions, such as compressibility and conduc- tivity, and screening (Thomas-Fermi, Debye). We will then discuss Landau Fermi-liquid theory, which will allow us understand why despite strong electron-electron interactions, nevertheless much of the phenomenology of a Fermi gas extends to a Fermi liquid. We will conclude with discussion of electrons on the lattice, treated within the Hubbard and t-J models and will study transition to a Mott insulator and magnetism 1 I. INTRODUCTION A. Outline electron gas ground state and excitations • thermodynamics • Pauli paramagnetism • Landau diamagnetism • Hartree-Fock theory of interactions: ground state energy • Stoner ferromagnetic instability • response functions • Landau Fermi-liquid theory • electrons on the lattice: Hubbard and t-J models • Mott insulators and magnetism • B. Background In these lectures, we will study itinerate electron liquid, namely metals. In principle a fully quantum mechanical, strongly Coulomb-interacting description is required. -

Intermediate Statistics in Thermoelectric Properties of Solids
Intermediate statistics in thermoelectric properties of solids André A. Marinho1, Francisco A. Brito1,2 1 Departamento de Física, Universidade Federal de Campina Grande, 58109-970 Campina Grande, Paraíba, Brazil and 2 Departamento de Física, Universidade Federal da Paraíba, Caixa Postal 5008, 58051-970 João Pessoa, Paraíba, Brazil (Dated: July 23, 2019) Abstract We study the thermodynamics of a crystalline solid by applying intermediate statistics manifested by q-deformation. We based part of our study on both Einstein and Debye models, exploring primarily de- formed thermal and electrical conductivities as a function of the deformed Debye specific heat. The results revealed that the q-deformation acts in two different ways but not necessarily as independent mechanisms. It acts as a factor of disorder or impurity, modifying the characteristics of a crystalline structure, which are phenomena described by q-bosons, and also as a manifestation of intermediate statistics, the B-anyons (or B-type systems). For the latter case, we have identified the Schottky effect, normally associated with high-Tc superconductors in the presence of rare-earth-ion impurities, and also the increasing of the specific heat of the solids beyond the Dulong-Petit limit at high temperature, usually related to anharmonicity of interatomic interactions. Alternatively, since in the q-bosons the statistics are in principle maintained the effect of the deformation acts more slowly due to a small change in the crystal lattice. On the other hand, B-anyons that belong to modified statistics are more sensitive to the deformation. PACS numbers: 02.20-Uw, 05.30-d, 75.20-g arXiv:1907.09055v1 [cond-mat.stat-mech] 21 Jul 2019 1 I. -

Chapter 4: Bonding in Solids and Electronic Properties
Chapter 4: Bonding in Solids and Electronic Properties Free electron theory Consider free electrons in a metal – an electron gas. •regards a metal as a box in which electrons are free to move. •assumes nuclei stay fixed on their lattice sites surrounded by core electrons, while the valence electrons move freely through the solid. •Ignoring the core electrons, one can treat the outer electrons with a quantum mechanical description. •Taking just one electron, the problem is reduced to a particle in a box. Electron is confined to a line of length a. Schrodinger equation 2 2 2 d d 2me E 2 (E V ) 2 2 2me dx dx Electron is not allowed outside the box, so the potential is ∞ outside the box. Energy is quantized, with quantum numbers n. 2 2 2 n2h2 h2 n n n In 1d: E In 3d: E a b c 2 8m a2 b2 c2 8mea e 1 2 2 2 2 Each set of quantum numbers n , n , h n n n a b a b c E 2 2 2 and nc will give rise to an energy level. 8me a b c •In three dimensions, there are multiple combination of energy levels that will give the same energy, whereas in one-dimension n and –n are equal in energy. 2 2 2 2 2 2 na/a nb/b nc/c na /a + nb /b + nc /c 6 6 6 108 The number of states with the 2 2 10 108 same energy is known as the degeneracy. 2 10 2 108 10 2 2 108 When dealing with a crystal with ~1020 atoms, it becomes difficult to work out all the possible combinations. -
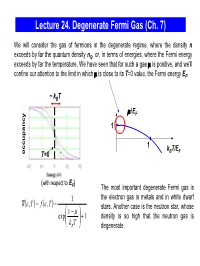
Lecture 24. Degenerate Fermi Gas (Ch
Lecture 24. Degenerate Fermi Gas (Ch. 7) We will consider the gas of fermions in the degenerate regime, where the density n exceeds by far the quantum density nQ, or, in terms of energies, where the Fermi energy exceeds by far the temperature. We have seen that for such a gas μ is positive, and we’ll confine our attention to the limit in which μ is close to its T=0 value, the Fermi energy EF. ~ kBT μ/EF 1 1 kBT/EF occupancy T=0 (with respect to E ) F The most important degenerate Fermi gas is 1 the electron gas in metals and in white dwarf nε()(),, T= f ε T = stars. Another case is the neutron star, whose ε⎛ − μ⎞ exp⎜ ⎟ +1 density is so high that the neutron gas is ⎝kB T⎠ degenerate. Degenerate Fermi Gas in Metals empty states ε We consider the mobile electrons in the conduction EF conduction band which can participate in the charge transport. The band energy is measured from the bottom of the conduction 0 band. When the metal atoms are brought together, valence their outer electrons break away and can move freely band through the solid. In good metals with the concentration ~ 1 electron/ion, the density of electrons in the electron states electron states conduction band n ~ 1 electron per (0.2 nm)3 ~ 1029 in an isolated in metal electrons/m3 . atom The electrons are prevented from escaping from the metal by the net Coulomb attraction to the positive ions; the energy required for an electron to escape (the work function) is typically a few eV. -

Chapter 13 Ideal Fermi
Chapter 13 Ideal Fermi gas The properties of an ideal Fermi gas are strongly determined by the Pauli principle. We shall consider the limit: k T µ,βµ 1, B � � which defines the degenerate Fermi gas. In this limit, the quantum mechanical nature of the system becomes especially important, and the system has little to do with the classical ideal gas. Since this chapter is devoted to fermions, we shall omit in the following the subscript ( ) that we used for the fermionic statistical quantities in the previous chapter. − 13.1 Equation of state Consider a gas ofN non-interacting fermions, e.g., electrons, whose one-particle wave- functionsϕ r(�r) are plane-waves. In this case, a complete set of quantum numbersr is given, for instance, by the three cartesian components of the wave vector �k and thez spin projectionm s of an electron: r (k , k , k , m ). ≡ x y z s Spin-independent Hamiltonians. We will consider only spin independent Hamiltonian operator of the type ˆ 3 H= �k ck† ck + d r V(r)c r†cr , �k � where thefirst and the second terms are respectively the kinetic and th potential energy. The summation over the statesr (whenever it has to be performed) can then be reduced to the summation over states with different wavevectork(p=¯hk): ... (2s + 1) ..., ⇒ r � �k where the summation over the spin quantum numberm s = s, s+1, . , s has been taken into account by the prefactor (2s + 1). − − 159 160 CHAPTER 13. IDEAL FERMI GAS Wavefunctions in a box. We as- sume that the electrons are in a vol- ume defined by a cube with sidesL x, Ly,L z and volumeV=L xLyLz. -

Electron-Electron Interactions(Pdf)
Contents 2 Electron-electron interactions 1 2.1 Mean field theory (Hartree-Fock) ................ 3 2.1.1 Validity of Hartree-Fock theory .................. 6 2.1.2 Problem with Hartree-Fock theory ................ 9 2.2 Screening ..................................... 10 2.2.1 Elementary treatment ......................... 10 2.2.2 Kubo formula ............................... 15 2.2.3 Correlation functions .......................... 18 2.2.4 Dielectric constant ............................ 19 2.2.5 Lindhard function ............................ 21 2.2.6 Thomas-Fermi theory ......................... 24 2.2.7 Friedel oscillations ............................ 25 2.2.8 Plasmons ................................... 27 2.3 Fermi liquid theory ............................ 30 2.3.1 Particles and holes ............................ 31 2.3.2 Energy of quasiparticles. ....................... 36 2.3.3 Residual quasiparticle interactions ................ 38 2.3.4 Local energy of a quasiparticle ................... 42 2.3.5 Thermodynamic properties ..................... 44 2.3.6 Quasiparticle relaxation time and transport properties. 46 2.3.7 Effective mass m∗ of quasiparticles ................ 50 0 Reading: 1. Ch. 17, Ashcroft & Mermin 2. Chs. 5& 6, Kittel 3. For a more detailed discussion of Fermi liquid theory, see G. Baym and C. Pethick, Landau Fermi-Liquid Theory : Concepts and Ap- plications, Wiley 1991 2 Electron-electron interactions The electronic structure theory of metals, developed in the 1930’s by Bloch, Bethe, Wilson and others, assumes that electron-electron interac- tions can be neglected, and that solid-state physics consists of computing and filling the electronic bands based on knowldege of crystal symmetry and atomic valence. To a remarkably large extent, this works. In simple compounds, whether a system is an insulator or a metal can be deter- mined reliably by determining the band filling in a noninteracting cal- culation.