Chapter 6 Free Electron Fermi
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Solid State Physics II Level 4 Semester 1 Course Content
Solid State Physics II Level 4 Semester 1 Course Content L1. Introduction to solid state physics - The free electron theory : Free levels in one dimension. L2. Free electron gas in three dimensions. L3. Electrical conductivity – Motion in magnetic field- Wiedemann-Franz law. L4. Nearly free electron model - origin of the energy band. L5. Bloch functions - Kronig Penney model. L6. Dielectrics I : Polarization in dielectrics L7 .Dielectrics II: Types of polarization - dielectric constant L8. Assessment L9. Experimental determination of dielectric constant L10. Ferroelectrics (1) : Ferroelectric crystals L11. Ferroelectrics (2): Piezoelectricity L12. Piezoelectricity Applications L1 : Solid State Physics Solid state physics is the study of rigid matter, or solids, ,through methods such as quantum mechanics, crystallography, electromagnetism and metallurgy. It is the largest branch of condensed matter physics. Solid-state physics studies how the large-scale properties of solid materials result from their atomic- scale properties. Thus, solid-state physics forms the theoretical basis of materials science. It also has direct applications, for example in the technology of transistors and semiconductors. Crystalline solids & Amorphous solids Solid materials are formed from densely-packed atoms, which interact intensely. These interactions produce : the mechanical (e.g. hardness and elasticity), thermal, electrical, magnetic and optical properties of solids. Depending on the material involved and the conditions in which it was formed , the atoms may be arranged in a regular, geometric pattern (crystalline solids, which include metals and ordinary water ice) , or irregularly (an amorphous solid such as common window glass). Crystalline solids & Amorphous solids The bulk of solid-state physics theory and research is focused on crystals. -

Competition Between Paramagnetism and Diamagnetism in Charged
Competition between paramagnetism and diamagnetism in charged Fermi gases Xiaoling Jian, Jihong Qin, and Qiang Gu∗ Department of Physics, University of Science and Technology Beijing, Beijing 100083, China (Dated: November 10, 2018) The charged Fermi gas with a small Lande-factor g is expected to be diamagnetic, while that with a larger g could be paramagnetic. We calculate the critical value of the g-factor which separates the dia- and para-magnetic regions. In the weak-field limit, gc has the same value both at high and low temperatures, gc = 1/√12. Nevertheless, gc increases with the temperature reducing in finite magnetic fields. We also compare the gc value of Fermi gases with those of Boltzmann and Bose gases, supposing the particle has three Zeeman levels σ = 1, 0, and find that gc of Bose and Fermi gases is larger and smaller than that of Boltzmann gases,± respectively. PACS numbers: 05.30.Fk, 51.60.+a, 75.10.Lp, 75.20.-g I. INTRODUCTION mK and 10 µK, respectively. The ions can be regarded as charged Bose or Fermi gases. Once the quantum gas Magnetism of electron gases has been considerably has both the spin and charge degrees of freedom, there studied in condensed matter physics. In magnetic field, arises the competition between paramagnetism and dia- the magnetization of a free electron gas consists of two magnetism, as in electrons. Different from electrons, the independent parts. The spin magnetic moment of elec- g-factor for different magnetic ions is diverse, ranging trons results in the paramagnetic part (the Pauli para- from 0 to 2. -

Solid State Physics 2 Lecture 5: Electron Liquid
Physics 7450: Solid State Physics 2 Lecture 5: Electron liquid Leo Radzihovsky (Dated: 10 March, 2015) Abstract In these lectures, we will study itinerate electron liquid, namely metals. We will begin by re- viewing properties of noninteracting electron gas, developing its Greens functions, analyzing its thermodynamics, Pauli paramagnetism and Landau diamagnetism. We will recall how its thermo- dynamics is qualitatively distinct from that of a Boltzmann and Bose gases. As emphasized by Sommerfeld (1928), these qualitative di↵erence are due to the Pauli principle of electons’ fermionic statistics. We will then include e↵ects of Coulomb interaction, treating it in Hartree and Hartree- Fock approximation, computing the ground state energy and screening. We will then study itinerate Stoner ferromagnetism as well as various response functions, such as compressibility and conduc- tivity, and screening (Thomas-Fermi, Debye). We will then discuss Landau Fermi-liquid theory, which will allow us understand why despite strong electron-electron interactions, nevertheless much of the phenomenology of a Fermi gas extends to a Fermi liquid. We will conclude with discussion of electrons on the lattice, treated within the Hubbard and t-J models and will study transition to a Mott insulator and magnetism 1 I. INTRODUCTION A. Outline electron gas ground state and excitations • thermodynamics • Pauli paramagnetism • Landau diamagnetism • Hartree-Fock theory of interactions: ground state energy • Stoner ferromagnetic instability • response functions • Landau Fermi-liquid theory • electrons on the lattice: Hubbard and t-J models • Mott insulators and magnetism • B. Background In these lectures, we will study itinerate electron liquid, namely metals. In principle a fully quantum mechanical, strongly Coulomb-interacting description is required. -
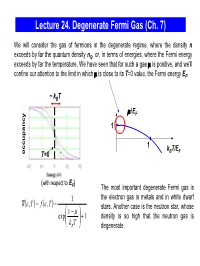
Lecture 24. Degenerate Fermi Gas (Ch
Lecture 24. Degenerate Fermi Gas (Ch. 7) We will consider the gas of fermions in the degenerate regime, where the density n exceeds by far the quantum density nQ, or, in terms of energies, where the Fermi energy exceeds by far the temperature. We have seen that for such a gas μ is positive, and we’ll confine our attention to the limit in which μ is close to its T=0 value, the Fermi energy EF. ~ kBT μ/EF 1 1 kBT/EF occupancy T=0 (with respect to E ) F The most important degenerate Fermi gas is 1 the electron gas in metals and in white dwarf nε()(),, T= f ε T = stars. Another case is the neutron star, whose ε⎛ − μ⎞ exp⎜ ⎟ +1 density is so high that the neutron gas is ⎝kB T⎠ degenerate. Degenerate Fermi Gas in Metals empty states ε We consider the mobile electrons in the conduction EF conduction band which can participate in the charge transport. The band energy is measured from the bottom of the conduction 0 band. When the metal atoms are brought together, valence their outer electrons break away and can move freely band through the solid. In good metals with the concentration ~ 1 electron/ion, the density of electrons in the electron states electron states conduction band n ~ 1 electron per (0.2 nm)3 ~ 1029 in an isolated in metal electrons/m3 . atom The electrons are prevented from escaping from the metal by the net Coulomb attraction to the positive ions; the energy required for an electron to escape (the work function) is typically a few eV. -

Chapter 13 Ideal Fermi
Chapter 13 Ideal Fermi gas The properties of an ideal Fermi gas are strongly determined by the Pauli principle. We shall consider the limit: k T µ,βµ 1, B � � which defines the degenerate Fermi gas. In this limit, the quantum mechanical nature of the system becomes especially important, and the system has little to do with the classical ideal gas. Since this chapter is devoted to fermions, we shall omit in the following the subscript ( ) that we used for the fermionic statistical quantities in the previous chapter. − 13.1 Equation of state Consider a gas ofN non-interacting fermions, e.g., electrons, whose one-particle wave- functionsϕ r(�r) are plane-waves. In this case, a complete set of quantum numbersr is given, for instance, by the three cartesian components of the wave vector �k and thez spin projectionm s of an electron: r (k , k , k , m ). ≡ x y z s Spin-independent Hamiltonians. We will consider only spin independent Hamiltonian operator of the type ˆ 3 H= �k ck† ck + d r V(r)c r†cr , �k � where thefirst and the second terms are respectively the kinetic and th potential energy. The summation over the statesr (whenever it has to be performed) can then be reduced to the summation over states with different wavevectork(p=¯hk): ... (2s + 1) ..., ⇒ r � �k where the summation over the spin quantum numberm s = s, s+1, . , s has been taken into account by the prefactor (2s + 1). − − 159 160 CHAPTER 13. IDEAL FERMI GAS Wavefunctions in a box. We as- sume that the electrons are in a vol- ume defined by a cube with sidesL x, Ly,L z and volumeV=L xLyLz. -

Inorganic Chemistry for Dummies® Published by John Wiley & Sons, Inc
Inorganic Chemistry Inorganic Chemistry by Michael L. Matson and Alvin W. Orbaek Inorganic Chemistry For Dummies® Published by John Wiley & Sons, Inc. 111 River St. Hoboken, NJ 07030-5774 www.wiley.com Copyright © 2013 by John Wiley & Sons, Inc., Hoboken, New Jersey Published by John Wiley & Sons, Inc., Hoboken, New Jersey Published simultaneously in Canada No part of this publication may be reproduced, stored in a retrieval system or transmitted in any form or by any means, electronic, mechanical, photocopying, recording, scanning or otherwise, except as permitted under Sections 107 or 108 of the 1976 United States Copyright Act, without either the prior written permis- sion of the Publisher, or authorization through payment of the appropriate per-copy fee to the Copyright Clearance Center, 222 Rosewood Drive, Danvers, MA 01923, (978) 750-8400, fax (978) 646-8600. Requests to the Publisher for permission should be addressed to the Permissions Department, John Wiley & Sons, Inc., 111 River Street, Hoboken, NJ 07030, (201) 748-6011, fax (201) 748-6008, or online at http://www.wiley. com/go/permissions. Trademarks: Wiley, the Wiley logo, For Dummies, the Dummies Man logo, A Reference for the Rest of Us!, The Dummies Way, Dummies Daily, The Fun and Easy Way, Dummies.com, Making Everything Easier, and related trade dress are trademarks or registered trademarks of John Wiley & Sons, Inc. and/or its affiliates in the United States and other countries, and may not be used without written permission. All other trade- marks are the property of their respective owners. John Wiley & Sons, Inc., is not associated with any product or vendor mentioned in this book. -

Chapter 6 Free Electron Fermi Gas
理学院 物理系 沈嵘 Chapter 6 Free Electron Fermi Gas 6.1 Electron Gas Model and its Ground State 6.2 Thermal Properties of Electron Gas 6.3 Free Electrons in Electric Fields 6.4 Hall Effect 6.5 Thermal Conductivity of Metals 6.6 Failures of the free electron gas model 1 6.1 Electron Gas Model and its Ground State 6.1 Electron Gas Model and its Ground State I. Basic Assumptions of Electron Gas Model Metal: valence electrons → conduction electrons (moving freely) ü The simplest metals are the alkali metals—lithium, sodium, 2 potassium, cesium, and rubidium. 6.1 Electron Gas Model and its Ground State density of electrons: Zr n = N m A A where Z is # of conduction electrons per atom, A is relative atomic mass, rm is the density of mass in the metal. The spherical volume of each electron is, 1 3 1 V 4 3 æ 3 ö = = p rs rs = ç ÷ n N 3 è 4p nø Free electron gas model: Suppose, except the confining potential near surfaces of metals, conduction electrons are completely free. The conduction electrons thus behave just like gas atoms in an ideal gas --- free electron gas. 3 6.1 Electron Gas Model and its Ground State Basic Properties: ü Ignore interactions of electron-ion type (free electron approx.) ü And electron-eletron type (independent electron approx). Total energy are of kinetic type, ignore potential energy contribution. ü The classical theory had several conspicuous successes 4 6.1 Electron Gas Model and its Ground State Long Mean Free Path: ü From many types of experiments it is clear that a conduction electron in a metal can move freely in a straight path over many atomic distances. -

Electron-Electron Interactions(Pdf)
Contents 2 Electron-electron interactions 1 2.1 Mean field theory (Hartree-Fock) ................ 3 2.1.1 Validity of Hartree-Fock theory .................. 6 2.1.2 Problem with Hartree-Fock theory ................ 9 2.2 Screening ..................................... 10 2.2.1 Elementary treatment ......................... 10 2.2.2 Kubo formula ............................... 15 2.2.3 Correlation functions .......................... 18 2.2.4 Dielectric constant ............................ 19 2.2.5 Lindhard function ............................ 21 2.2.6 Thomas-Fermi theory ......................... 24 2.2.7 Friedel oscillations ............................ 25 2.2.8 Plasmons ................................... 27 2.3 Fermi liquid theory ............................ 30 2.3.1 Particles and holes ............................ 31 2.3.2 Energy of quasiparticles. ....................... 36 2.3.3 Residual quasiparticle interactions ................ 38 2.3.4 Local energy of a quasiparticle ................... 42 2.3.5 Thermodynamic properties ..................... 44 2.3.6 Quasiparticle relaxation time and transport properties. 46 2.3.7 Effective mass m∗ of quasiparticles ................ 50 0 Reading: 1. Ch. 17, Ashcroft & Mermin 2. Chs. 5& 6, Kittel 3. For a more detailed discussion of Fermi liquid theory, see G. Baym and C. Pethick, Landau Fermi-Liquid Theory : Concepts and Ap- plications, Wiley 1991 2 Electron-electron interactions The electronic structure theory of metals, developed in the 1930’s by Bloch, Bethe, Wilson and others, assumes that electron-electron interac- tions can be neglected, and that solid-state physics consists of computing and filling the electronic bands based on knowldege of crystal symmetry and atomic valence. To a remarkably large extent, this works. In simple compounds, whether a system is an insulator or a metal can be deter- mined reliably by determining the band filling in a noninteracting cal- culation. -

Schedule for Rest of Quarter • Review: 3D Free Electron Gas • Heat Capacity
Lecture 16 Housekeeping: schedule for rest of quarter Review: 3D free electron gas Heat capacity of free electron gas Semi classical treatment of electric conductivity Review: 3D fee electron gas This model ignores the lattice and only treats the electrons in a metal. It assumes that electrons do not interact with each other except for pauli exclusion. 풌∙풓 Wavelike-electrons defined by their momentum eigenstate k: 휓풌(풓) = 푒 Treat electrons like quantum particle in a box: confined to a 3D box of dimensions L in every direction Periodic boundary conditions on wavefunction: 휓(푥 + 퐿, 푦, 푧) = 휓(푥, 푦, 푧) 휓(푥, 푦 + 퐿, 푧) = 휓(푥, 푦, 푧) 휓(푥, 푦, 푧 + 퐿) = 휓(푥, 푦, 푧) Permissible values of k of the form: 2휋 4휋 푘 = 0, ± , ± , … 푥 퐿 퐿 Free electron schrodinger’s eqn in 3D + boundary conditions gives energy eigenvalues: ℏ2 휕2 휕2 휕2 − ( + + ) 휓 (푟) = 휖 휓 (푟) 2푚 휕푥2 휕푦2 휕푧2 푘 푘 푘 ℏ2 ℏ2푘2 (푘2 + 푘2 + 푘2) = 휖 = 2푚 푥 푦 푧 푘 2푚 There are N electrons and we put them into available eigenstates following these rules: o Every state is defined by a unique quantized value of (푘푥, 푘푦, 푘푧) o Every state can hold one spin up and one spin down electron o Assume that single-electron solution above applies to collection of electrons (i.e. electrons do not interact and change the eigenstates) o Fill low energy states first. In 3D, this corresponds to filling up a sphere in k space, one 2 2 2 2 ‘shell’ at a time. -

Fermi and Bose Gases
Fermi and Bose gases Anders Malthe-Sørenssen 25. november 2013 1 1 Thermodynamic identities and Legendre transforms 2 Fermi-Dirac and Bose-Einstein distribution When we discussed the ideal gas we assumed that quantum effects were not important. This was implied when we introduced the term 1=N! for the partition function for the ideal gas, because this term only was valid in the limit when the number of states are many compared to the number of particles. Now, we will address the general case. We start from an individual particle. For a single particle, we address its orbitals: Its possible quantum states, each of which corresponds to a solution of the Schrodinger equation for a single particle. For many particles we will address how the “fill up" the various orbitals { where the underlying assump- tion is that we can treat a many-particle system as a system with the same energy levels as for a single particular system, that is as a system with no interactions between the states of the various particles. However, the number of particles that can occupy the same state depends on the type of particle: For Fermions (1/2 spin) Only 0 or 1 fermion can be in a particular state. For Bosons (integer spin) Any number of bosons can be in a particular state. We will now apply a Gibbs ensemble approach to study this system. This means that we will consider a system where µ, V; T is constant. We can use the Gibbs sum formalism to address the behavior of this system. -
![Arxiv:2006.09236V4 [Quant-Ph] 27 May 2021](https://docslib.b-cdn.net/cover/1529/arxiv-2006-09236v4-quant-ph-27-may-2021-1001529.webp)
Arxiv:2006.09236V4 [Quant-Ph] 27 May 2021
The Free Electron Gas in Cavity Quantum Electrodynamics Vasil Rokaj,1, ∗ Michael Ruggenthaler,1, y Florian G. Eich,1 and Angel Rubio1, 2, z 1Max Planck Institute for the Structure and Dynamics of Matter, Center for Free Electron Laser Science, 22761 Hamburg, Germany 2Center for Computational Quantum Physics (CCQ), Flatiron Institute, 162 Fifth Avenue, New York NY 10010 (Dated: May 31, 2021) Cavity modification of material properties and phenomena is a novel research field largely mo- tivated by the advances in strong light-matter interactions. Despite this progress, exact solutions for extended systems strongly coupled to the photon field are not available, and both theory and experiments rely mainly on finite-system models. Therefore a paradigmatic example of an exactly solvable extended system in a cavity becomes highly desireable. To fill this gap we revisit Som- merfeld's theory of the free electron gas in cavity quantum electrodynamics (QED). We solve this system analytically in the long-wavelength limit for an arbitrary number of non-interacting elec- trons, and we demonstrate that the electron-photon ground state is a Fermi liquid which contains virtual photons. In contrast to models of finite systems, no ground state exists if the diamagentic A2 term is omitted. Further, by performing linear response we show that the cavity field induces plasmon-polariton excitations and modifies the optical and the DC conductivity of the electron gas. Our exact solution allows us to consider the thermodynamic limit for both electrons and photons by constructing an effective quantum field theory. The continuum of modes leads to a many-body renormalization of the electron mass, which modifies the fermionic quasiparticle excitations of the Fermi liquid and the Wigner-Seitz radius of the interacting electron gas. -

Impact of the Interband Transitions in Gold and Silver on the Dynamics of Propagating and Localized Surface Plasmons
nanomaterials Article Impact of the Interband Transitions in Gold and Silver on the Dynamics of Propagating and Localized Surface Plasmons Krystyna Kolwas * and Anastasiya Derkachova Institute of Physics, Polish Academy of Sciences, Al. Lotnikow 32/46, 02-668 Warsaw, Poland; [email protected] * Correspondence: [email protected]; Tel.: +48-22-116-33-19 Received: 19 June 2020; Accepted: 14 July 2020; Published: 19 July 2020 Abstract: Understanding and modeling of a surface-plasmon phenomenon on lossy metals interfaces based on simplified models of dielectric function lead to problems when confronted with reality. For a realistic description of lossy metals, such as gold and silver, in the optical range of the electromagnetic spectrum and in the adjacent spectral ranges it is necessary to account not only for ohmic losses but also for the radiative losses resulting from the frequency-dependent interband transitions. We give a detailed analysis of Surface Plasmon Polaritons (SPPs) and Localized Surface Plasmons (LPSs) supported by such realistic metal/dielectric interfaces based on the dispersion relations both for flat and spherical gold and silver interfaces in the extended frequency and nanoparticle size ranges. The study reveals the region of anomalous dispersion for a silver flat interface in the near UV spectral range and high-quality factors for larger nanoparticles. We show that the frequency-dependent interband transition accounted in the dielectric function in a way allowing reproducing well the experimentally measured indexes of refraction does exert the pronounced impact not only on the properties of SPP and LSP for gold interfaces but also, with the weaker but not negligible impact, on the corresponding silver interfaces in the optical ranges and the adjacent spectral ranges.