Phys 446 Solid State Physics Lecture 7 (Ch. 4.1 – 4.3, 4.6.) Last Time
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Fermi- Dirac Statistics
Prof. Surajit Dhara Guest Teacher, Dept. Of Physics, Narajole Raj College GE2T (Thermal physics and Statistical Mechanics) , Topic :- Fermi- Dirac Statistics Fermi- Dirac Statistics Basic features: The basic features of the Fermi-Dirac statistics: 1. The particles are all identical and hence indistinguishable. 2. The fermions obey (a) the Heisenberg’s uncertainty relation and also (b) the exclusion principle of Pauli. 3. As a consequence of 2(a), there exists a number of quantum states for a given energy level and because of 2(b) , there is a definite a priori restriction on the number of fermions in a quantum state; there can be simultaneously no more than one particle in a quantum state which would either remain empty or can at best contain one fermion. If , again, the particles are isolated and non-interacting, the following two additional condition equations apply to the system: ∑ ; Thermodynamic probability: Consider an isolated system of N indistinguishable, non-interacting particles obeying Pauli’s exclusion principle. Let particles in the system have energies respectively and let denote the degeneracy . So the number of distinguishable arrangements of particles among eigenstates in the ith energy level is …..(1) Therefore, the thermodynamic probability W, that is, the total number of s eigenstates of the whole system is the product of ……(2) GE2T (Thermal physics and Statistical Mechanics), Topic :- Fermi- Dirac Statistics: Circulated by-Prof. Surajit Dhara, Dept. Of Physics, Narajole Raj College FD- distribution function : Most probable distribution : From eqn(2) above , taking logarithm and applying Stirling’s theorem, For this distribution to represent the most probable distribution , the entropy S or klnW must be maximum, i.e. -

Density of States Information from Low Temperature Specific Heat
JOURNAL OF RESE ARC H of th e National Bureau of Standards - A. Physics and Chemistry Val. 74A, No.3, May-June 1970 Density of States I nformation from Low Temperature Specific Heat Measurements* Paul A. Beck and Helmut Claus University of Illinois, Urbana (October 10, 1969) The c a lcul ati on of one -electron d ensit y of s tate va lues from the coeffi cient y of the te rm of the low te mperature specifi c heat lin ear in te mperature is compli cated by many- body effects. In parti c ul ar, the electron-p honon inte raction may enhance the measured y as muc h as tw ofo ld. The e nha nce me nt fa ctor can be eva luat ed in the case of supe rconducting metals and a ll oys. In the presence of magneti c mo ments, add it ional complicati ons arise. A magneti c contribution to the measured y was ide ntifi e d in the case of dilute all oys and a lso of concentrated a lJ oys wh e re parasiti c antife rromagnetis m is s upe rim posed on a n over-a ll fe rromagneti c orde r. No me thod has as ye t bee n de vised to e valu ate this magne ti c part of y. T he separati on of the te mpera ture- li near term of the s pec ifi c heat may itself be co mpli cated by the a ppearance of a s pecific heat a no ma ly due to magneti c cluste rs in s upe rpa ramagneti c or we ak ly ferromagneti c a ll oys. -

Solid State Physics II Level 4 Semester 1 Course Content
Solid State Physics II Level 4 Semester 1 Course Content L1. Introduction to solid state physics - The free electron theory : Free levels in one dimension. L2. Free electron gas in three dimensions. L3. Electrical conductivity – Motion in magnetic field- Wiedemann-Franz law. L4. Nearly free electron model - origin of the energy band. L5. Bloch functions - Kronig Penney model. L6. Dielectrics I : Polarization in dielectrics L7 .Dielectrics II: Types of polarization - dielectric constant L8. Assessment L9. Experimental determination of dielectric constant L10. Ferroelectrics (1) : Ferroelectric crystals L11. Ferroelectrics (2): Piezoelectricity L12. Piezoelectricity Applications L1 : Solid State Physics Solid state physics is the study of rigid matter, or solids, ,through methods such as quantum mechanics, crystallography, electromagnetism and metallurgy. It is the largest branch of condensed matter physics. Solid-state physics studies how the large-scale properties of solid materials result from their atomic- scale properties. Thus, solid-state physics forms the theoretical basis of materials science. It also has direct applications, for example in the technology of transistors and semiconductors. Crystalline solids & Amorphous solids Solid materials are formed from densely-packed atoms, which interact intensely. These interactions produce : the mechanical (e.g. hardness and elasticity), thermal, electrical, magnetic and optical properties of solids. Depending on the material involved and the conditions in which it was formed , the atoms may be arranged in a regular, geometric pattern (crystalline solids, which include metals and ordinary water ice) , or irregularly (an amorphous solid such as common window glass). Crystalline solids & Amorphous solids The bulk of solid-state physics theory and research is focused on crystals. -

Fermi Surface of Copper Electrons That Do Not Interact
StringString theorytheory andand thethe mysteriousmysterious quantumquantum mattermatter ofof condensedcondensed mattermatter physics.physics. Jan Zaanen 1 String theory: what is it really good for? - Hadron (nuclear) physics: quark-gluon plasma in RIHC. - Quantum matter: quantum criticality in heavy fermion systems, high Tc superconductors, … Started in 2001, got on steam in 2007. Son Hartnoll Herzog Kovtun McGreevy Liu Schalm 2 Quantum critical matter Quark gluon plasma Iron High Tc Heavy fermions superconductors superconductors (?) Quantum critical Quantum critical 3 High-Tc Has Changed Landscape of Condensed Matter Physics High-resolution ARPES Magneto-optics Transport-Nernst effect Spin-polarized Neutron STM High Tc Superconductivity Inelastic X-Ray Scattering Angle-resolved MR/Heat Capacity ? Photoemission spectrum Hairy Black holes … 6 Holography and quantum matter But first: crash course in holography “Planckian dissipation”: quantum critical matter at high temperature, perfect fluids and the linear resistivity (Son, Policastro, …, Sachdev). Reissner Nordstrom black hole: “critical Fermi-liquids”, like high Tc’s normal state (Hong Liu, John McGreevy). Dirac hair/electron star: Fermi-liquids emerging from a non Fermi liquid (critical) ultraviolet, like overdoped high Tc (Schalm, Cubrovic, Hartnoll). Scalar hair: holographic superconductivity, a new mechanism for superconductivity at a high temperature (Hartnoll, Herzog,Horowitz) . 7 General relativity “=“ quantum field theory Gravity Quantum fields Maldacena 1997 = 8 Anti de Sitter-conformal -

Quantum Mechanics Electromotive Force
Quantum Mechanics_Electromotive force . Electromotive force, also called emf[1] (denoted and measured in volts), is the voltage developed by any source of electrical energy such as a batteryor dynamo.[2] The word "force" in this case is not used to mean mechanical force, measured in newtons, but a potential, or energy per unit of charge, measured involts. In electromagnetic induction, emf can be defined around a closed loop as the electromagnetic workthat would be transferred to a unit of charge if it travels once around that loop.[3] (While the charge travels around the loop, it can simultaneously lose the energy via resistance into thermal energy.) For a time-varying magnetic flux impinging a loop, theElectric potential scalar field is not defined due to circulating electric vector field, but nevertheless an emf does work that can be measured as a virtual electric potential around that loop.[4] In a two-terminal device (such as an electrochemical cell or electromagnetic generator), the emf can be measured as the open-circuit potential difference across the two terminals. The potential difference thus created drives current flow if an external circuit is attached to the source of emf. When current flows, however, the potential difference across the terminals is no longer equal to the emf, but will be smaller because of the voltage drop within the device due to its internal resistance. Devices that can provide emf includeelectrochemical cells, thermoelectric devices, solar cells and photodiodes, electrical generators,transformers, and even Van de Graaff generators.[4][5] In nature, emf is generated whenever magnetic field fluctuations occur through a surface. -

Chapter 4: Bonding in Solids and Electronic Properties
Chapter 4: Bonding in Solids and Electronic Properties Free electron theory Consider free electrons in a metal – an electron gas. •regards a metal as a box in which electrons are free to move. •assumes nuclei stay fixed on their lattice sites surrounded by core electrons, while the valence electrons move freely through the solid. •Ignoring the core electrons, one can treat the outer electrons with a quantum mechanical description. •Taking just one electron, the problem is reduced to a particle in a box. Electron is confined to a line of length a. Schrodinger equation 2 2 2 d d 2me E 2 (E V ) 2 2 2me dx dx Electron is not allowed outside the box, so the potential is ∞ outside the box. Energy is quantized, with quantum numbers n. 2 2 2 n2h2 h2 n n n In 1d: E In 3d: E a b c 2 8m a2 b2 c2 8mea e 1 2 2 2 2 Each set of quantum numbers n , n , h n n n a b a b c E 2 2 2 and nc will give rise to an energy level. 8me a b c •In three dimensions, there are multiple combination of energy levels that will give the same energy, whereas in one-dimension n and –n are equal in energy. 2 2 2 2 2 2 na/a nb/b nc/c na /a + nb /b + nc /c 6 6 6 108 The number of states with the 2 2 10 108 same energy is known as the degeneracy. 2 10 2 108 10 2 2 108 When dealing with a crystal with ~1020 atoms, it becomes difficult to work out all the possible combinations. -
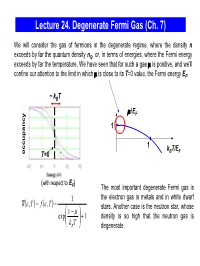
Lecture 24. Degenerate Fermi Gas (Ch
Lecture 24. Degenerate Fermi Gas (Ch. 7) We will consider the gas of fermions in the degenerate regime, where the density n exceeds by far the quantum density nQ, or, in terms of energies, where the Fermi energy exceeds by far the temperature. We have seen that for such a gas μ is positive, and we’ll confine our attention to the limit in which μ is close to its T=0 value, the Fermi energy EF. ~ kBT μ/EF 1 1 kBT/EF occupancy T=0 (with respect to E ) F The most important degenerate Fermi gas is 1 the electron gas in metals and in white dwarf nε()(),, T= f ε T = stars. Another case is the neutron star, whose ε⎛ − μ⎞ exp⎜ ⎟ +1 density is so high that the neutron gas is ⎝kB T⎠ degenerate. Degenerate Fermi Gas in Metals empty states ε We consider the mobile electrons in the conduction EF conduction band which can participate in the charge transport. The band energy is measured from the bottom of the conduction 0 band. When the metal atoms are brought together, valence their outer electrons break away and can move freely band through the solid. In good metals with the concentration ~ 1 electron/ion, the density of electrons in the electron states electron states conduction band n ~ 1 electron per (0.2 nm)3 ~ 1029 in an isolated in metal electrons/m3 . atom The electrons are prevented from escaping from the metal by the net Coulomb attraction to the positive ions; the energy required for an electron to escape (the work function) is typically a few eV. -

Chapter 13 Ideal Fermi
Chapter 13 Ideal Fermi gas The properties of an ideal Fermi gas are strongly determined by the Pauli principle. We shall consider the limit: k T µ,βµ 1, B � � which defines the degenerate Fermi gas. In this limit, the quantum mechanical nature of the system becomes especially important, and the system has little to do with the classical ideal gas. Since this chapter is devoted to fermions, we shall omit in the following the subscript ( ) that we used for the fermionic statistical quantities in the previous chapter. − 13.1 Equation of state Consider a gas ofN non-interacting fermions, e.g., electrons, whose one-particle wave- functionsϕ r(�r) are plane-waves. In this case, a complete set of quantum numbersr is given, for instance, by the three cartesian components of the wave vector �k and thez spin projectionm s of an electron: r (k , k , k , m ). ≡ x y z s Spin-independent Hamiltonians. We will consider only spin independent Hamiltonian operator of the type ˆ 3 H= �k ck† ck + d r V(r)c r†cr , �k � where thefirst and the second terms are respectively the kinetic and th potential energy. The summation over the statesr (whenever it has to be performed) can then be reduced to the summation over states with different wavevectork(p=¯hk): ... (2s + 1) ..., ⇒ r � �k where the summation over the spin quantum numberm s = s, s+1, . , s has been taken into account by the prefactor (2s + 1). − − 159 160 CHAPTER 13. IDEAL FERMI GAS Wavefunctions in a box. We as- sume that the electrons are in a vol- ume defined by a cube with sidesL x, Ly,L z and volumeV=L xLyLz. -

Extent of Fermi-Surface Reconstruction in the High-Temperature Superconductor Hgba2cuo4+Δ
Extent of Fermi-surface reconstruction in the high-temperature superconductor HgBa2CuO4+δ Mun K. Chana,1, Ross D. McDonalda, Brad J. Ramshawd, Jon B. Bettsa, Arkady Shekhterb, Eric D. Bauerc, and Neil Harrisona aPulsed Field Facility, National High Magnetic Field Laboratory, Los Alamos National Laboratory, Los Alamos, New Mexico 87545, USA; bNational High Magnetic Field Laboratory, Florida State University, Tallahassee, Forida 32310, USA; cLos Alamos National Laboratory, Los Alamos, New Mexico, 87545, USA; dLaboratory of Atomic and Solid State Physics, Cornell University, Ithaca, NY 148.3, USA This manuscript was compiled on June 17, 2020 High magnetic fields have revealed a surprisingly small Fermi- change at TH ≈ 20 K. These results attest to the high-quality surface in underdoped cuprates, possibly resulting from Fermi- of the present crystals and confirm prior results indicating surface reconstruction due to an order parameter that breaks trans- Fermi-surface reconstruction in Hg1201 at similar doping lev- lational symmetry of the crystal lattice. A crucial issue concerns els (6,9, 10). the doping extent of this state and its relationship to the principal We have also found a peak in the planar resistivity ρ(T ) pseudogap and superconducting phases. We employ pulsed mag- at high fields, Figs.1C&D. ρ(T ) upturns at a temperature netic field measurements on the cuprate HgBa2CuO4+δ to identify coincident with the steep downturn in Rxy(T ), as shown in signatures of Fermi surface reconstruction from a sign change of the Fig.1D and SI Appendix Fig. S7. The common character- Hall effect and a peak in the temperature-dependent planar resistiv- istic temperatures suggest the features in ρ(T ) are related ity. -

Inorganic Chemistry for Dummies® Published by John Wiley & Sons, Inc
Inorganic Chemistry Inorganic Chemistry by Michael L. Matson and Alvin W. Orbaek Inorganic Chemistry For Dummies® Published by John Wiley & Sons, Inc. 111 River St. Hoboken, NJ 07030-5774 www.wiley.com Copyright © 2013 by John Wiley & Sons, Inc., Hoboken, New Jersey Published by John Wiley & Sons, Inc., Hoboken, New Jersey Published simultaneously in Canada No part of this publication may be reproduced, stored in a retrieval system or transmitted in any form or by any means, electronic, mechanical, photocopying, recording, scanning or otherwise, except as permitted under Sections 107 or 108 of the 1976 United States Copyright Act, without either the prior written permis- sion of the Publisher, or authorization through payment of the appropriate per-copy fee to the Copyright Clearance Center, 222 Rosewood Drive, Danvers, MA 01923, (978) 750-8400, fax (978) 646-8600. Requests to the Publisher for permission should be addressed to the Permissions Department, John Wiley & Sons, Inc., 111 River Street, Hoboken, NJ 07030, (201) 748-6011, fax (201) 748-6008, or online at http://www.wiley. com/go/permissions. Trademarks: Wiley, the Wiley logo, For Dummies, the Dummies Man logo, A Reference for the Rest of Us!, The Dummies Way, Dummies Daily, The Fun and Easy Way, Dummies.com, Making Everything Easier, and related trade dress are trademarks or registered trademarks of John Wiley & Sons, Inc. and/or its affiliates in the United States and other countries, and may not be used without written permission. All other trade- marks are the property of their respective owners. John Wiley & Sons, Inc., is not associated with any product or vendor mentioned in this book. -

Chapter 6 Free Electron Fermi Gas
理学院 物理系 沈嵘 Chapter 6 Free Electron Fermi Gas 6.1 Electron Gas Model and its Ground State 6.2 Thermal Properties of Electron Gas 6.3 Free Electrons in Electric Fields 6.4 Hall Effect 6.5 Thermal Conductivity of Metals 6.6 Failures of the free electron gas model 1 6.1 Electron Gas Model and its Ground State 6.1 Electron Gas Model and its Ground State I. Basic Assumptions of Electron Gas Model Metal: valence electrons → conduction electrons (moving freely) ü The simplest metals are the alkali metals—lithium, sodium, 2 potassium, cesium, and rubidium. 6.1 Electron Gas Model and its Ground State density of electrons: Zr n = N m A A where Z is # of conduction electrons per atom, A is relative atomic mass, rm is the density of mass in the metal. The spherical volume of each electron is, 1 3 1 V 4 3 æ 3 ö = = p rs rs = ç ÷ n N 3 è 4p nø Free electron gas model: Suppose, except the confining potential near surfaces of metals, conduction electrons are completely free. The conduction electrons thus behave just like gas atoms in an ideal gas --- free electron gas. 3 6.1 Electron Gas Model and its Ground State Basic Properties: ü Ignore interactions of electron-ion type (free electron approx.) ü And electron-eletron type (independent electron approx). Total energy are of kinetic type, ignore potential energy contribution. ü The classical theory had several conspicuous successes 4 6.1 Electron Gas Model and its Ground State Long Mean Free Path: ü From many types of experiments it is clear that a conduction electron in a metal can move freely in a straight path over many atomic distances. -

Bulk Fermi Surface Coexistence with Dirac Surface State in Bi2se3: a Comparison of Photoemission and Shubnikov–De Haas Measurements
PHYSICAL REVIEW B 81, 205407 ͑2010͒ Bulk Fermi surface coexistence with Dirac surface state in Bi2Se3: A comparison of photoemission and Shubnikov–de Haas measurements James G. Analytis,1,2 Jiun-Haw Chu,1,2 Yulin Chen,1,2 Felipe Corredor,1,2 Ross D. McDonald,3 Z. X. Shen,1,2 and Ian R. Fisher1,2 1Stanford Institute for Materials and Energy Sciences, SLAC National Accelerator Laboratory, 2575 Sand Hill Road, Menlo Park, California 94025, USA 2Geballe Laboratory for Advanced Materials and Department of Applied Physics, Stanford University, Stanford, California 94305, USA 3Los Alamos National Laboratory, Los Alamos, New Mexico 87545, USA ͑Received 2 February 2010; published 5 May 2010͒ Shubnikov-de Haas ͑SdH͒ oscillations and angle-resolved photoemission spectroscopy ͑ARPES͒ are used to probe the Fermi surface of single crystals of Bi2Se3. We find that SdH and ARPES probes quantitatively agree on measurements of the effective mass and bulk band dispersion. In high carrier density samples, the two probes also agree in the exact position of the Fermi level EF, but for lower carrier density samples discrepan- cies emerge in the position of EF. In particular, SdH reveals a bulk three-dimensional Fermi surface for samples with carrier densities as low as 1017 cm−3. We suggest a simple mechanism to explain these differ- ences and discuss consequences for existing and future transport studies of topological insulators. DOI: 10.1103/PhysRevB.81.205407 PACS number͑s͒: 72.20.My, 03.65.Vf, 71.70.Di, 73.25.ϩi Recently, a new state of matter, known as a topological high level of consistency across all samples measured from insulator, has been predicted to exist in a number of materi- the same batch.