Eliminating Preventable Harm and Improving the Quality of Health Care Delivery
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
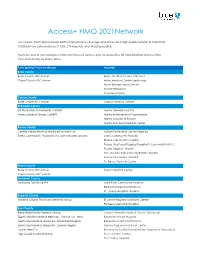
Access+ HMO 2021Network
Access+ HMO 2021Network Our Access+ HMO plan provides both comprehensive coverage and access to a high-quality network of more than 10,000 primary care physicians (PCPs), 270 hospitals, and 34,000 specialists. You have zero or low copayments for most covered services, plus no deductible for hospitalization or preventive care and virtually no claims forms. Participating Physician Groups Hospitals Butte County Butte County BSC Admin Enloe Medical Center Cohasset Glenn County BSC Admin Enloe Medical Center Esplanade Enloe Rehabilitation Center Orchard Hospital Oroville Hospital Colusa County Butte County BSC Admin Colusa Medical Center El Dorado County Hill Physicians Sacramento CalPERS Mercy General Hospital Mercy Medical Group CalPERS Methodist Hospital of Sacramento Mercy Hospital of Folsom Mercy San Juan Medical Center Fresno County Central Valley Medical Medical Providers Inc. Adventist Medical Center Reedley Sante Community Physicians Inc. Sante Health Systems Clovis Community Hospital Fresno Community Hospital Fresno Heart and Surgical Hospital A Community RMCC Fresno Surgical Hospital San Joaquin Valley Rehabilitation Hospital Selma Community Hospital St. Agnes Medical Center Glenn County Butte County BSC Admin Glenn Medical Center Glenn County BSC Admin Humboldt County Humboldt Del Norte IPA Mad River Community Hospital Redwood Memorial Hospital St. Joseph Hospital - Eureka Imperial County Imperial County Physicians Medical Group El Centro Regional Medical Center Pioneers Memorial Hospital Kern County Bakersfield Family Medical -

Systemwide Emergency Management Status Report
Systemwide Emergency Management Status Report UC Systemwide Emergency Management Status Report i Table of Contents Introduction ...................................................................................................................................................... 1 Systemwide Summary of Conformity with NFPA Emergency Management Standard Criteria ... 2 ERMIS Emergency Management Key Performance Indicator (KPI) ..................................................... 7 Individual Program Executive Summaries ................................................................................................. 8 Berkeley ........................................................................................................................................................ 8 Lawrence Berkeley National Laboratory ............................................................................................... 9 Davis ............................................................................................................................................................10 Davis Health System ................................................................................................................................11 Irvine ............................................................................................................................................................12 Irvine Health System ...............................................................................................................................13 Los -

2019 a Glimpse Into Data…Your Data 1111 W
2019 A GLIMPSE INTO DATA…YOUR DATA 1111 W. la Palma Ave. Anaheim, CA 92801 714.774.1450 Hospital Type Provider Number Short Term Acute 050226 Care Hospital NPI Number Ownership 1891938122 Proprietary - Corporation Medical School Affiliation CBSA Code No Affiliation 31080 Los Angeles Accrediation Agency Long Beach The Joint Commission Anaheim CA TABLE OF CONTENTS Hospital Financials 6 Hospital Payments and Volumes 39 Payor Information 48 Physicians Output Facts 57 Market Share Technology 90 Procedures and Departments 241 by the Numbers Hospital Assessments 263 Definitions 279 5 HOSPITAL FINANCIALS 6 Assets NATIONAL CURRENT ASSETS 06/30/2018 06/30/2018 CBSA BENCHMARK BENCHMARK CASH ON HAND $27,543,217.00 $29,021,469.00 $15,506,901.00 $28,385,971.00 TEMPORARY INVESTMENTS $37,017,480.00 $98,436,246.00 ACCOUNTS RECEIVABLE $210,780,518.00 $213,989,159.00 $55,238,855.00 $149,598,728.00 OTHER RECEIVABLES $8,545,418.00 $37,738,512.00 ALLOWANCES FOR UNCOLLECTIBLE ($183,776,393.00) ($185,804,256.00) ($40,577,959.00) ($115,106,758.00) INVENTORY $3,083,556.00 $2,928,396.00 $3,219,045.00 $3,554,690.00 PREPAID EXPENSES $8,089,670.00 $2,044,988.00 $1,756,147.00 $7,729,992.00 OTHER CURRENT ASSETS $11,147,847.00 $31,890,540.00 TOTAL CURRENT ASSETS $65,720,568.00 $62,179,756.00 $73,295,404.00 $156,603,010.00 Financial Percentages and Ratios NATIONAL PERCENTAGES 06/30/2019 CBSA BENCHMARK BENCHMARK NET OPERATING PROFIT MARGIN -3.5% -3.3% -21.8% NET INCOME MARGIN -2.9% 5.2% -9.8% BAD DEBT TO NET PATIENT REVENUE RATIO 5.8% 6.5% 3.1% BAD DEBT TO ACCOUNTS RECEIVABLE RATIO 5.9% 19.6% 5.1% PATIENT DISCOUNT PCT. -

San Diego House Staff Association: Proposals 2018-2021
San Diego House Staff Association: Proposals 2018-2021 Page | 0 TABLE OF CONTENTS Contents INTRODUCTION / BACKGROUND ................................................................................................................. 1 A. 2018 Negotiating Committee ........................................................................................................... 1 B. Procedure ......................................................................................................................................... 1 C. Definitions ........................................................................................................................................ 1 D. Overview / Background ................................................................................................................... 1 E. The Cost of Living & The Dilemma for House Staff ......................................................................... 4 PROPOSALS ................................................................................................................................................... 6 FELLOWS ....................................................................................................................................................... 6 A. Background ....................................................................................................................................... 6 B. The Fellows’ Representatives ......................................................................................................... -

VASDHS Psychology Internship Brochure
2021-22 UCSD/VA PSYCHOLOGY INTERNSHIP TRAINING PROGRAM Department of Psychiatry University of California, San Diego VA San Diego Healthcare System Co-Directors Sandra Brown, Ph.D., ABPP Amy Jak, Ph.D. Applicant Manual Last updated September 2020 Dear Prospective Applicant, Thank you for your interest in the UCSD/VA Psychology Internship Training Program. In the following pages, you will find detailed information about our internship, including clinical training, didactic experiences, research opportunities, our faculty, and application instructions. Our program is based on the scientist-practitioner model. As such, we seek competitive applicants interested and experienced in both research and clinical practice, particularly those interested in academic careers. Clinical training and didactic experiences integrate cutting-edge evidence-based techniques with a foundation of established empirically-supported treatments and assessment. We also recognize the importance of diversity represented by our trainees and faculty, as well as in our patients. We encourage those of diverse backgrounds, in all the many ways that diversity is defined, to apply to our program. Our full-time internship has been accredited by APA since 1986 (Further information about accreditation of this program can be found at: Office of Program Consultation and Accreditation, American Psychological Association, 750 First Street, N.E., Washington, DC 20002-4242, Phone: (202) 336-5979, Fax: (202) 336-5978, Email: [email protected], Web: www.apa.org/ed/accreditation). For the 2021-2022 year, interns will earn an annual stipend of $29,212. Our competitive benefits, both for UCSD and for the VA, include health insurance, paid leave days, and paid holidays. The COVID-19 pandemic has led our faculty to implement multiple changes in our training program. -
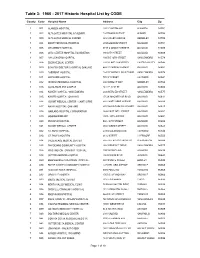
Table 3: 1960 - 2017 Historic Hospital List by CODE
Table 3: 1960 - 2017 Historic Hospital List by CODE County Code Hospital Name Address City Zip 1 001 ALAMEDA HOSPITAL 2070 CLINTON AVE ALAMEDA 94501 1 002 ALTA BATES HOSPITAL AT ALBANY 1247 MARIN AVENUE ALBANY 94706 1 003 ALTA BATES MEDICAL CENTER 2450 ASHBY AVENUE BERKELEY 94705 1 004 BOOTH MEMORIAL HOSPITAL 2794 GARDEN STREET OAKLAND 94701 1 005 CHILDREN'S HOSPITAL 51ST & GROVE STREETS OAKLAND 94609 1 006 CIVIC CENTER HOSPITAL FOUNDATION 390 40TH STREET OAKLAND 94609 1 007 SAN LEANDRO HOSPITAL 13855 E 14TH STREET SAN LEANDRO 94578 1 008 EDEN MEDICAL CENTER 20103 LAKE CHABOT RD CASTRO VALLEY 94546 1 009 ESKATON DOCTORS HOSPITAL OAKLAND 4600 E FAIRFAX AVENUE OAKLAND 94601 1 010 FAIRMONT HOSPITAL 15400 FOOTHILL BOULEVARD SAN LEANDRO 94578 1 011 HAYWARD HOSPITAL 770 'A' STREET HAYWARD 94541 1 012 HERRICK MEMORIAL HOSPITAL 2001 DWIGHT WAY BERKELEY 94704 1 013 ACMC-HIGHLAND CAMPUS 1411 E. 31ST ST OAKLAND 94602 1 014 KAISER HOSPITAL: SAN LEANDRO 2500 MERCED STREET SAN LEANDRO 94577 1 015 KAISER HOSPITAL: OAKLAND 275 W. MACARTHUR BLVD OAKLAND 94611 1 016 SUMMIT MEDICAL CENTER - HAWTHORNE 350 HAWTHORNE AVENUE OAKLAND 94609 1 017 NAVAL HOSPITAL: OAKLAND 8750 MOUNTAIN BOULEVARD OAKLAND 94627 1 018 OAKLAND HOSPITAL CORPORATION 2648 EAST 14TH STREET OAKLAND 94601 1 019 OGORMAN INFANT 2587 - 35TH AVENUE OAKLAND 94601 1 020 PERALTA HOSPITAL 450 - 30TH STREET OAKLAND 94609 1 021 SUMMIT MEDICAL CENTER 3100 SUMMIT STREET OAKLAND 94623 1 022 ST. ROSE HOSPITAL 27200 CALAROGA AVE HAYWARD 94540 1 023 ST. PAUL'S HOSPITAL 813 J STREET LIVERMORE 94550 1 024 VALLEYCARE MEDICAL CENTER 5555 W. -

USA Customers
USA Customers ▪ Acadia General Hospital - Crowley, LA ▪ Advocate BroMenn Healthcare Hospitals - Normal, IL ▪ Advocate Christ Medical Center - Oak Lawn, IL ▪ Advocate Condell Medical Center - Libertyville, IL ▪ Advocate Good Samaritan Hospital - Downer’s Grove, IL ▪ Advocate Good Shepherd Hospital - Barrington, IL ▪ Advocate Illinois Masonic Medical Center - Chicago, IL ▪ Advocate Lutheran General Hospital - Park Ridge, IL ▪ Advocate Sherman Hospital - Elgin, IL ▪ Advocate South Suburban Hospital - Hazel Crest, IL ▪ Advocate Trinity Hospital - Chicago, IL ▪ Akron Children's Hospital - Akron, OH ▪ Alamance Regional Medical Center - Burlington, NC ▪ Alameda County Medical Center - Oakland, CA ▪ Alaska Native Medical Center - Anchorage, AK ▪ Albany Medical Center Hospital - Albany, NY ▪ Albany Stratton VA Medical Center - Albany, NY ▪ Allina, Abbott Northwestern Hospital - Plymouth, MN ▪ Allina, Buffalo Hospital - Buffalo, MN ▪ Allina, Cambridge Medical Center - Cambridge, MN ▪ Allina, District One Hospital - Faribault, MN ▪ Allina, Mercy Hospital - Coon Rapids, MN ▪ Allina, Owatonna Hospital - Owatonna, MN ▪ Allina, Philips Eye Institute - Minneapolis, MN ▪ Allina, Regina Medical Center - Hastings, MN ▪ Allina, River Falls Area Hospital - River Falls, MN ▪ Allina, St. Francis Regional Medical Center - Shakopee, MN ▪ Allina, United Hospital - St. Paul, MN ▪ Allina, Unity Hospital - Fridley, MN ▪ Annie Penn Hospital - Reidsville, NC ▪ Apollo Care, LLC - Columbia, MO ▪ Appleton Medical Center - Appleton, WI ▪ Ashland Hospital Corp Kings Daughter -

Planetree Partners CERTIFICATION* ORGANIZATION LOCATION
Planetree Partners CERTIFICATION* ORGANIZATION LOCATION CIMED Argentina Hospital Universitario Austral Argentina Centro de Rehabilitacion Quantum Argentina Hospital Universitario Austral Argentina Paseo Champagnat Argentina Sede Escobar Argentina Sede Lujan Argentina Sede Officia Argentina Sede San Miguel Argentina Aurrum Australia Aurrum – Healesville Australia Aurrum – Reservoir Australia Children’s Health Queensland Hospital and Health Service Australia Child and Youth Community Health Service Australia Child and Youth Mental Health Service Australia Lady Cilento Children’s Hospital Australia Logan Central Community Health Centre Australia Metro South Health Australia Beaudesert Hospital Australia Beenleigh Community Health Centre Australia Browns Plains Community Health Centre Australia Corinda Community Health Centre Australia Eight Mile Plains Community Health Centre Australia WWW.PLANETREE.ORG 1 Planetree Partners CERTIFICATION* ORGANIZATION LOCATION Inala Community Health Centre Australia Logan Hospital Australia Marie Rod Community Health Centre Australia Princess Alexandra Hospital Australia Queen Elizabeth II Jubilee Hospital Australia Redland Community Health Centre Australia Redland Hospital Australia Wynnum Community Health Centre Australia Associação De Assistência A Criança Deficiente (AACD) Brazil Hospital Anchieta Brazil Hospital Marcelino Champagnat Brazil Hospital Mater Dei SA Brazil Mãe de Deus (Mother of God Hospital) Brazil Moinhos de Vento Hospital Brazil Sociedade Beneficente Israelita Brasileira Albert Einstein -

University of California, San Diego Annual Financial Report 2009–10
UNIVERSITY OF CALIFORNIA, SAN DIEGO ANNUAL FINANCIAL REPORT 2009–10 UNIVERSITY OF CALIFORNIA, SAN DIEGO ANNUAL FINANCIAL REPORT 2009–10 1 Chancellor Fox Awarded the NATIONAL MEDAL OF SCIENCE UC SAN DIEGO CHANCELLOR MARYE ANNE FOX received the National Medal of Science in 2010, the highest honor bestowed by the United States government on scientists, engineers, and inventors. A nationally recognized organic chemist and academic leader, Fox has been elected to membership in the National Academy of Sciences and the American Philosophical Society, and to fellowships in the American Academy of Arts and Sciences and the American Association for the Advance- ment of Science. She has also received honorary degrees from twelve U.S. institutions. Her research has focused on fundamental principles that were later translated into practical use in solar energy conversion, environmental remediation, and materials science. Fox is the most recent member of the UC San Diego community to receive this prestigious award. Previous living National Medal of Science recipients from UC San Diego are E. Margaret Burbidge, astrophysics (1983); Walter Munk, geophysics (1983); Michael H. Freedman, mathematics (1987); Yuan-Cheng Fung, bioen- gineering (2000); Andrew Viterbi, electrical and computer engineering (2008); and Craig Venter, pharmacology (2009). Clockwise from top: Chancellor Marye Anne Fox; Fox receives the medal from President Barack Obama at the White House, November 17, 2010; an inspirational note Fox wrote as a young girl “I always thought I would be a scientist. Once you’ve understood something that didn’t exist before, it’s almost like you have to figure out what the answer to the next question is, and generate the next question after that. -

UC San Diego Health Patients, As Well As Patients Seen Throughout UC Health System
Testimony of Dr. Charles Daniels House Energy and Commerce Committee 340B Oversight Hearing, July 11, 2018 Introduction Good morning, Chairman Burgess and Chairman Walden, Ranking Member Green, and Ranking Member Pallone. Thank you for this opportunity to share my experience with the 340B Drug Pricing Program. I want to also want to say hello to Congressman Peters, my own Congressman, who serves on this Committee, along with Congresswoman Matsui, who represents the people of our sister institution UC Davis Health. I have been able to personally share with Congressman Peters and Congresswoman Matsui the value of the 340B discount to UC San Diego Health patients, as well as patients seen throughout UC Health System. My name is Dr. Charles Daniels, and I serve as Pharmacist-In-Chief for the University of California San Diego’s academic medical center, referred to as UC San Diego Health. As Pharmacist-In-Chief, I oversee UC San Diego Health’s administration and use of the 340B Program. Who is UC San Diego Health? UC San Diego Health is a public academic medical center serving the people of San Diego and surrounding communities. The medical center’s service imprint extends over 100 miles into remote El Centro in Imperial County. Our mission is to deliver outstanding patient care through commitment to the community, groundbreaking research, and inspired teaching. UC San Diego Health, a premier provider of tertiary and quaternary services, is comprised of three major inpatient facilities, the Hillcrest Medical Center, the Jacobs Medical Center, and the Sulpizio Cardiovascular Center, along with the region’s only National Cancer Institute (NCI)-designated Comprehensive Cancer Center. -

2019 Capital Financial Plan
Attachment 1 Capital Financial Plan 2019-25 University of California Office of the President Capital Asset Strategies & Finance 1111 Franklin Street, 6th Floor Oakland, California 94607-5200 Cover photo: UC Berkeley Photo credit: Elena Zhukova 2019-25 CAPITAL FINANCIAL PLAN TABLE OF CONTENTS Summary 5 CAPITAL PLAN BY LOCATION How to Read the Tables 17 Berkeley 19 Davis 27 UC Davis Health 33 Irvine 39 UC Irvine Health 47 Los Angeles 53 UC Los Angeles Health 58 Merced 63 Riverside 69 San Diego 75 UC San Diego Health 83 San Francisco 89 UCSF Health 94 Santa Barbara 99 Santa Cruz 107 Division of Agriculture and Natural Resources 115 Lawrence Berkeley National Laboratory 119 Systemwide and Office of the President 125 Appendix – Projects of Interest to UC Health 130 2019-25 CAPITAL FINANCIAL PLAN 4 SUMMARY The University’s capital program is driven by the campuses’ and medical centers’ academic and strategic plans. The Capital Financial Plan (CFP) is developed based on the needs at each location for buildings and other physical infrastructure to achieve these overarching plans. ▪ Strategic and Academic Plans define priority areas and goals and may include institutional aspirations. ▪ The Long Range Development Plan is a comprehensive plan, as approved by the Regents, on proposed future physical planning and development of a campus or medical center. ▪ The Physical Design Framework identifies planning principles and objectives for design of the physical environment. The CFP presents proposed capital projects, public private partnerships, and acquisition of real property that support these plans. The 2019-25 CFP represents $52 billion of capital need as articulated by the campuses and medical centers over this year and the next five fiscal years (through 2024-25). -

Top-40-Arch-Firms.Pdf
JANUARY 26, 2015 LOS ANGELES BUSINESS JOURNAL 17 NEXT WeeK ARCHITECTURE FIRMS The Largest Motion Picture Distributors THE LIST Ranked by 2014 L.A. County billings and Largest Production Companies Rank Company L.A. County Current Projects Profile Top Local Executive THE PACESETTER: Gensler • name Billings1 (partial list) • L.A. architects • name tops the list of the largest • address • 2014 • L.A. employees2 • title architecture firms operating • website • 2013 • offices (L.A./total) • phone in L.A. County with $81 • headquarters million in billings last year. Gensler $81.1 Television Academy, Sony, LAX Midfield, Element LA, 125 John Adams/Barbara Bouza/ That’s an increase of $5.1 500 S. Figueroa St. $76.0 Metropolis, NBC Universal, JPL/NASA, Wiseburn High 279 Michael White million from 2013. The 1 Los Angeles 90071 School, Waldorf Astoria, California State University 1/46 Co-Managing Directors firm employs 279 people gensler.com N/A (213) 327-3600 dedicated to its architecture RTKL Associates Inc. 49.4 Grand Avenue Hotel and Residences, LXM Residential 47 Nate Cherry practice, including 125 2 333 S. Hope St., Suite C200 31.9 Towers, W Hotel Beijing, Eau Claire Mixed Use, ARC 232 Vice President licensed architects, in its Los Angeles 90071 Centre Plaza, Wuxi Suning 1/15 (213) 633-6000 downtown Los Angeles rtkl.com Baltimore office. Aecom Technology Corp. 38.0 New Face of the Central Terminal Area, Midfield Satellite 13 Ross Wimer 515 S. Flower St. 16.5 Concourse, San Diego Airport Parking Plaza, Downtown 26 Senior Vice President 3 Los Angeles 90071 Harbor, Glendale Narrows Phase 1, San Pedro 14/923 (213) 593-8000 aecom.com/architecture Waterfront Master Plan Los Angeles ZGF Architects 35.1 California Science Center, UCLA Wasserman Football 30 Ted Hyman 515 S.