2014 CPQCC Manual of Definitions Final
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
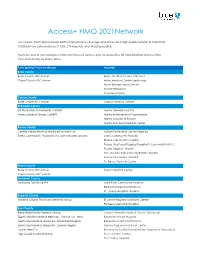
Access+ HMO 2021Network
Access+ HMO 2021Network Our Access+ HMO plan provides both comprehensive coverage and access to a high-quality network of more than 10,000 primary care physicians (PCPs), 270 hospitals, and 34,000 specialists. You have zero or low copayments for most covered services, plus no deductible for hospitalization or preventive care and virtually no claims forms. Participating Physician Groups Hospitals Butte County Butte County BSC Admin Enloe Medical Center Cohasset Glenn County BSC Admin Enloe Medical Center Esplanade Enloe Rehabilitation Center Orchard Hospital Oroville Hospital Colusa County Butte County BSC Admin Colusa Medical Center El Dorado County Hill Physicians Sacramento CalPERS Mercy General Hospital Mercy Medical Group CalPERS Methodist Hospital of Sacramento Mercy Hospital of Folsom Mercy San Juan Medical Center Fresno County Central Valley Medical Medical Providers Inc. Adventist Medical Center Reedley Sante Community Physicians Inc. Sante Health Systems Clovis Community Hospital Fresno Community Hospital Fresno Heart and Surgical Hospital A Community RMCC Fresno Surgical Hospital San Joaquin Valley Rehabilitation Hospital Selma Community Hospital St. Agnes Medical Center Glenn County Butte County BSC Admin Glenn Medical Center Glenn County BSC Admin Humboldt County Humboldt Del Norte IPA Mad River Community Hospital Redwood Memorial Hospital St. Joseph Hospital - Eureka Imperial County Imperial County Physicians Medical Group El Centro Regional Medical Center Pioneers Memorial Hospital Kern County Bakersfield Family Medical -

2019 a Glimpse Into Data…Your Data 1111 W
2019 A GLIMPSE INTO DATA…YOUR DATA 1111 W. la Palma Ave. Anaheim, CA 92801 714.774.1450 Hospital Type Provider Number Short Term Acute 050226 Care Hospital NPI Number Ownership 1891938122 Proprietary - Corporation Medical School Affiliation CBSA Code No Affiliation 31080 Los Angeles Accrediation Agency Long Beach The Joint Commission Anaheim CA TABLE OF CONTENTS Hospital Financials 6 Hospital Payments and Volumes 39 Payor Information 48 Physicians Output Facts 57 Market Share Technology 90 Procedures and Departments 241 by the Numbers Hospital Assessments 263 Definitions 279 5 HOSPITAL FINANCIALS 6 Assets NATIONAL CURRENT ASSETS 06/30/2018 06/30/2018 CBSA BENCHMARK BENCHMARK CASH ON HAND $27,543,217.00 $29,021,469.00 $15,506,901.00 $28,385,971.00 TEMPORARY INVESTMENTS $37,017,480.00 $98,436,246.00 ACCOUNTS RECEIVABLE $210,780,518.00 $213,989,159.00 $55,238,855.00 $149,598,728.00 OTHER RECEIVABLES $8,545,418.00 $37,738,512.00 ALLOWANCES FOR UNCOLLECTIBLE ($183,776,393.00) ($185,804,256.00) ($40,577,959.00) ($115,106,758.00) INVENTORY $3,083,556.00 $2,928,396.00 $3,219,045.00 $3,554,690.00 PREPAID EXPENSES $8,089,670.00 $2,044,988.00 $1,756,147.00 $7,729,992.00 OTHER CURRENT ASSETS $11,147,847.00 $31,890,540.00 TOTAL CURRENT ASSETS $65,720,568.00 $62,179,756.00 $73,295,404.00 $156,603,010.00 Financial Percentages and Ratios NATIONAL PERCENTAGES 06/30/2019 CBSA BENCHMARK BENCHMARK NET OPERATING PROFIT MARGIN -3.5% -3.3% -21.8% NET INCOME MARGIN -2.9% 5.2% -9.8% BAD DEBT TO NET PATIENT REVENUE RATIO 5.8% 6.5% 3.1% BAD DEBT TO ACCOUNTS RECEIVABLE RATIO 5.9% 19.6% 5.1% PATIENT DISCOUNT PCT. -

Planetree Partners CERTIFICATION* ORGANIZATION LOCATION
Planetree Partners CERTIFICATION* ORGANIZATION LOCATION CIMED Argentina Hospital Universitario Austral Argentina Centro de Rehabilitacion Quantum Argentina Hospital Universitario Austral Argentina Paseo Champagnat Argentina Sede Escobar Argentina Sede Lujan Argentina Sede Officia Argentina Sede San Miguel Argentina Aurrum Australia Aurrum – Healesville Australia Aurrum – Reservoir Australia Children’s Health Queensland Hospital and Health Service Australia Child and Youth Community Health Service Australia Child and Youth Mental Health Service Australia Lady Cilento Children’s Hospital Australia Logan Central Community Health Centre Australia Metro South Health Australia Beaudesert Hospital Australia Beenleigh Community Health Centre Australia Browns Plains Community Health Centre Australia Corinda Community Health Centre Australia Eight Mile Plains Community Health Centre Australia WWW.PLANETREE.ORG 1 Planetree Partners CERTIFICATION* ORGANIZATION LOCATION Inala Community Health Centre Australia Logan Hospital Australia Marie Rod Community Health Centre Australia Princess Alexandra Hospital Australia Queen Elizabeth II Jubilee Hospital Australia Redland Community Health Centre Australia Redland Hospital Australia Wynnum Community Health Centre Australia Associação De Assistência A Criança Deficiente (AACD) Brazil Hospital Anchieta Brazil Hospital Marcelino Champagnat Brazil Hospital Mater Dei SA Brazil Mãe de Deus (Mother of God Hospital) Brazil Moinhos de Vento Hospital Brazil Sociedade Beneficente Israelita Brasileira Albert Einstein -

Access+ HMO Plan Overview Access+ HMO
Access+ HMO plan Overview Access+ HMO Now with lower rates! life is limitless. never stop. blueshieldca.com/calpers 1 You don’t have to sacrifice choice for lower costs Nothing matters more than your health. Except protecting it. Get access to one of the largest networks of doctors, specialists and hospitals in California with Access+ HMO®. It offers you many care options, so you can choose what’s right for you and your family. For 2018, we've worked to lower our rates for Access+ HMO to offer you a plan that delivers choice and affordability. Here are some of the valuable Access+ HMO programs and services that offer you more choice, convenience and control over your own care: Self-refer to a Select a different Contact a Stay covered across the specialist within primary care physician through country for extended your medical physician (PCP) Teladoc 24/7/365 periods with the Away group, without for each member when your doctor From Home Care® going to your of your family and isn’t available, program when you doctor first.1 change that doctor even while have students, long-term anytime you want. traveling, for only travelers and families a $5 copay.10 living apart.7 2 Access+ HMO plan Overview About the Access+ HMO plan Blue Shield’s Access+ HMO plan is designed to provide both comprehensive coverage and access to one of the largest, quality networks of doctors, specialists and hospitals throughout the state. With our Access+ HMO plan, you can expect zero or low copayments for most covered services, plus no deductible and virtually no claim forms. -

OSHPD FACILITY CODES -- Sorted by Hospital CPQCC Centers Indicated in Bold Italics
OSHPD FACILITY CODES -- Sorted by Hospital CPQCC Centers Indicated in Bold Italics OSHPD CENTER NAME CITY COUNTY 700564 30TH MEDICAL GROUP (700564) 700597 60TH MEDICAL GROUP (700597) 700431 722ND MEDICAL GROUP (700431) 700103 95TH MEDICAL GROUP (700103) 190323 ADVENTIST HEALTH - GLENDALE (190323) GLENDALE LOS ANGELES 190878 ADVENTIST HEALTH - WHITE MEMORIAL (190878) LOS ANGELES LOS ANGELES 150788 ADVENTIST HEALTH BAKERSFIELD (150788) BAKERSFIELD KERN 171049 ADVENTIST HEALTH CLEARLAKE (171049) CLEARLAKE LAKE 040875 ADVENTIST HEALTH FEATHER RIVER (040875) PARADISE BUTTE 164029 ADVENTIST HEALTH HANFORD (164029) HANFORD KINGS 390923 ADVENTIST HEALTH LODI MEMORIAL (390923) LODI SAN JOAQUIN 150808 ADVENTIST HEALTH MEDICAL CENTER TEHACHAPI VALLEY (150808) TEHACHAPI KERN 560525 ADVENTIST HEALTH SIMI VALLEY (560525) SIMI VALLEY VENTURA 554011 ADVENTIST HEALTH SONORA - GREENLEY (554011) SONORA TUOLUMNE 281078 ADVENTIST HEALTH ST. HELENA (281078) ST. HELENA NAPA 231396 ADVENTIST HEALTH UKIAH VALLEY (231396) UKIAH MENDOCINO 160787 ADVENTIST MEDICAL CENTER - CENTRAL VALLEY (160787) 100797 ADVENTIST MEDICAL CENTER - REEDLEY (100797) REEDLEY FRESNO 100793 ADVENTIST MEDICAL CENTER-SELMA (100793) SELMA FRESNO 301098 AHMC ANAHEIM REGIONAL MEDICAL CENTER (301098) ANAHEIM ORANGE 010735 ALAMEDA HOSPITAL (010735) ALAMEDA ALAMEDA 010989 ALAMEDA HOSPITAL AT WATERS EDGE (010989) ALAMEDA ALAMEDA 190017 ALHAMBRA HOSPITAL MEDICAL CENTER (190017) ALHAMBRA LOS ANGELES 010844 ALTA BATES SUMMIT MED CTR-HERRICK CAMPUS (010844) BERKELEY ALAMEDA 010937 ALTA BATES SUMMIT -

Eliminating Preventable Harm and Improving the Quality of Health Care Delivery
Collaborative Healthcare Patient Safety Organization Eliminating preventable harm and improving the quality of health care delivery ■ A DIVISION OF THE HOSPITAL QUALITY INSTITUTE ABOUT US CHPSO Mission WA ND MN OR Eliminating WI preventable IA NE NV OH harm and CO CA KS improving the KY TN AZ quality of NM AR GA health care TX delivery. HW CHPSO Vision CHPSO Membership remained steady in 2019 with more than 450 members in 13 states: Arkansas, CHPSO’s members Arizona, California, Georgia, Hawaii, Iowa, New Mexico, Nevada, Ohio, Oregon, Tennessee, Texas will lead the nation in and Washington. A total of 93 new facilities/organizations from seven states are pending or in progress. These members contributed to a growing database of more than 2.5 million safety events. providing the safest and highest quality Participate health care CHPSO works with members to facilitate and streamline the data submission process. CHPSO partners with NextPlane Solutions to allow members to connect to the CHPSO database, saving hospitals the cost of specialized solutions. With NextPlane Solutions, members can generate an Excel spreadsheet or plain text report delimited by commas or other characters. The entire process, including taxonomy mapping, generally takes up to three hours for the initial submission. For more information, contact CHPSO at [email protected] or visit our website, www.chpso.org. Benefits y Patient Safety Work Product y Event feedback and consultation (PSWP) privilege y Educational webinars y Collaborate and problem solve y Alerts and quarterly newsletters with other providers y Legal counsel discussion group y Periodic safety event evaluations y Job board y Bi-weekly Safe Table meetings y Custom research requests EVENT REPORTS MEDICATIONS 2019 events by category Medication Safety Event Report Analysis Other/Uncategorized The CHPSO database Overview of events in the dataset. -

Sharp Healthcare Community Benefit Plan and RePort Fiscal Year 2015
Sharp HealthCare Community Benefit Plan and Re port Fiscal Year 2015 ~ Committed to Improving the Health and Well-being of the Community ~ Sharp HealthCare Community Benefit Plan and Report Fiscal Year 2015 Submitted to: Office of Statewide Health Planning and Development Healthcare Information Division – Accounting and Reporting Systems Section 400 R Street, Room 250 Sacramento, CA 95811 Contents Section Description Page Preface ................................................................................................ i Glossary of Terms and Abbreviations ................................................ iii 1 An Overview of Sharp HealthCare......................................................1 2 Executive Summary .......................................................................... 49 3 Community Benefit Planning Process .............................................. 57 4 Sharp Chula Vista Medical Center.................................................... 65 5 Sharp Coronado Hospital and Healthcare Center ............................ 95 6 Sharp Grossmont Hospital ............................................................. 111 7 Sharp HospiceCare…………………………………………………….163 8 Sharp Metropolitan Medical Campus……………………………...….185 9 Sharp Mary Birch Hospital for Women & Newborns ....................... 187 10 Sharp Memorial Hospital ................................................................ 205 11 Sharp Mesa Vista Hospital and Sharp McDonald Center ............... 245 12 Sharp Health Plan ......................................................................... -
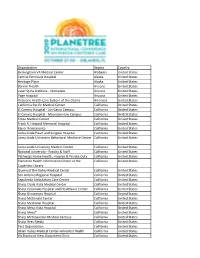
Organizations Who Have Attended Planetree.Xlsx
Organization Region Country Birmingham VA Medical Center Alabama United States Central Peninsula Hospital Alaska United States Heritage Place Alaska United States Banner Health Arizona United States Laser Spine Institute ‐ Scottsdale Arizona United States Page Hospital Arizona United States Veterans Health Care System of the Ozarks Arkansas United States California Pacific Medical Center California United States El Camino Hospital ‐ Los Gatos Campus California United States El Camino Hospital ‐ Mountainview Campus California United States Enloe Medical Center California United States Frank R. Howard Memorial Hospital California United States Kaiser Permanente California United States Loma Linda Heart and Surgical Hospital California United States Loma Linda University Behavioral Medicine Center California United States Loma Linda University Medical Center California United States National University ‐ Faculty & Staff California United States Pathways Home Health, Hospice & Private Duty California United States Planetree Health Information Center at the California United States Cupertino Library Queen of the Valley Medical Center California United States San Antonio Regional Hospital California United States Sepulveda Ambulatory Care Center California United States Sharp Chula Vista Medical Center California United States Sharp Coronado Hospital and Healthcare Center California United States Sharp Grossmont Hospital California United States Sharp McDonald Center California United States Sharp Memorial Hospital California United States Sharp -

Healthcare Equality Index 2016 Promoting Equitable and Inclusive Care for Lesbian, Gay, Bisexual and Transgender Patients and Their Families
Healthcare Equality Index 2016 Promoting Equitable and Inclusive Care for Lesbian, Gay, Bisexual and Transgender Patients and Their Families 2,060of the nation’s healthcare facilities rated on their commitment to LGBT equality and inclusion Why the HEI? Number of the nation’s healthcare To help facilities rated in the 2016 HEI by state patients find 32 Alabama 13 Alaska LGBT friendly 44 Arizona 29 Arkansas healthcare 210 California 30 Colorado facilities 22 Connecticut 10 Delaware 11 District of Columbia 105 Florida Turn the page 49 Georgia 12 Hawaii to see which 11 Idaho 71 Illinois of these 33 Indiana 25 Iowa facilities are 28 Kansas 24 Kentucky near you. 37 Louisiana 13 Maine 40 Maryland 69 Massachusetts 38 Michigan In addition to being a 54 Minnesota valuable tool and resource 33 Mississippi 44 Missouri for healthcare facilities, 12 Montana the HEI is used by LGBT 26 Nebraska patients, their loved ones 22 Nevada and allies to find facilities 12 New Hampshire that provide equitable and 27 New Jersey 23 New Mexico inclusive care. The ratings 115 New York for each participating and 43 North Carolina researched facility are 8 North Dakota published in this report, 87 Ohio available on our website 22 Oklahoma 50 Oregon and promoted to HRC’s 53 Pennsylvania over 1.5 million supporters. 9 Puerto Rico Consumers can easily 11 Rhode Island search our interactive map 24 South Carolina to see how facilities near 10 South Dakota 44 Tennessee them rate — giving patients 131 Texas the ability to choose where 42 Utah they would like to receive 7 Vermont care in their time of need. -
It'sthe Future
I t’s right. I t’s smart. I t’s the future. About CHPSO The California Hospital Patient Safety Organization is one of the oldest and largest PSOs in the nation. CHPSO analyzes patient safety data, develops and shares best practices, and helps individual hospitals accelerate safety improvements. Dedicated to patient safety, CHPSO works to help hospitals in their quest to eliminate preventable harm. CHPSO collaborates closely with the California Hospital Association and the Regional Hospital Associations to ensure an integrated approach. PARTICIPATION IN CHPSO OFFERS MANY BENEFITS • Extensive legal protections available under federal law • Data collection and analysis of medical errors and near misses • Access to the collective intelligence of patient safety experts and innovators • Confidential and collaborative learning environments • In-depth patient safety resources: website, newsletter, alerts, and customized research • Event investigation and response process improvement tools Why join CHPSO? It’s the right thing to do “ As an individual hospital, CHPSO assists hospitals in making their institutions safer by examining membership in CHPSO is a what went wrong and why, sharing lessons learned with other hospitals. must: available expertise on Joining CHPSO gives hospitals unprecedented access to incident data patient safety, peer review analysis for accelerating improvement. of Root Cause Analysis (RCA) Together we will eliminate preventable harm and ultimately shared reliable It’s the smart thing to do data will be a substantial CHPSO maximizes the full potential of data hospitals have collected. benet for our patient safety CHPSO members gain extraordinary access to incident data analysis and improvements.” the collective intelligence of patient safety experts nationwide. -

Clinical Agency Affiliation List
UNIVERSITY OF SOUTH ALABAMA COLLEGE OF NURSING December 17, 2018 Clinical Agency Affiliations and Locations Agency Name Programs Agency Requirements Covered 1st Operations Medical Group MSN, DNP, Post Student must complete Attachment 1 Graduate Certificate, Hurlburt Field, Florida MSN to DNP 2D Medical Group MSN Student must complete the Trainee Agreement Addendum Barksdale AFB, Louisiana 3HC Home Health & Hospice Care Undergraduate and Graduate Students Goldsboro, North Carolina 4th Medical Group Seymour Johnson AFB, North Carolina 5th Medical Group-Minot AFB MSN and DNP Student must complete Trainee Addendum. Student may utilize agreement for a period of no Minot AFB, North Dakota more than 2 years at a time. 18th Medical Group, Kadena Air Nurse Practitioner Student must complete the Trainee Agreement Addendum Base Japan 21st Medical Group, Peterson Air Executive Nursing Executive Nursing Admin DNP ONLY Admin DNP ONLY Force Base Student must complete Attachment 1 Colorado 35th Medical Group, Misawa AFB Advanced Practice Student must complete Trainee Agreement Addendum Japan 48th Medical Group, Executive Nsg Please contact the following for interest in this site. Admin DNP ONY RAF ALANE C. GARLISI, Lt Col, USAF, NC Lakenhealth , UK Education and Training Flight Commander 48th Medical Group, RAF Lakenheath, UK [email protected] Student must complete the Trainee Agreement. 52nd Medical Group MSN and DNP Student will need to complete Attachments 1&2 from Agency. Spangdahlem AFB, Germany 99th Medical Group Advanced Practice Student must complete Trainee Agreement Addendum Nellis Air Force Base, Nevada 354th Medical Group, Eielson, Nurse Practitioner Student: Must complete the Trainee Agreement Addendum, provided by the facility.