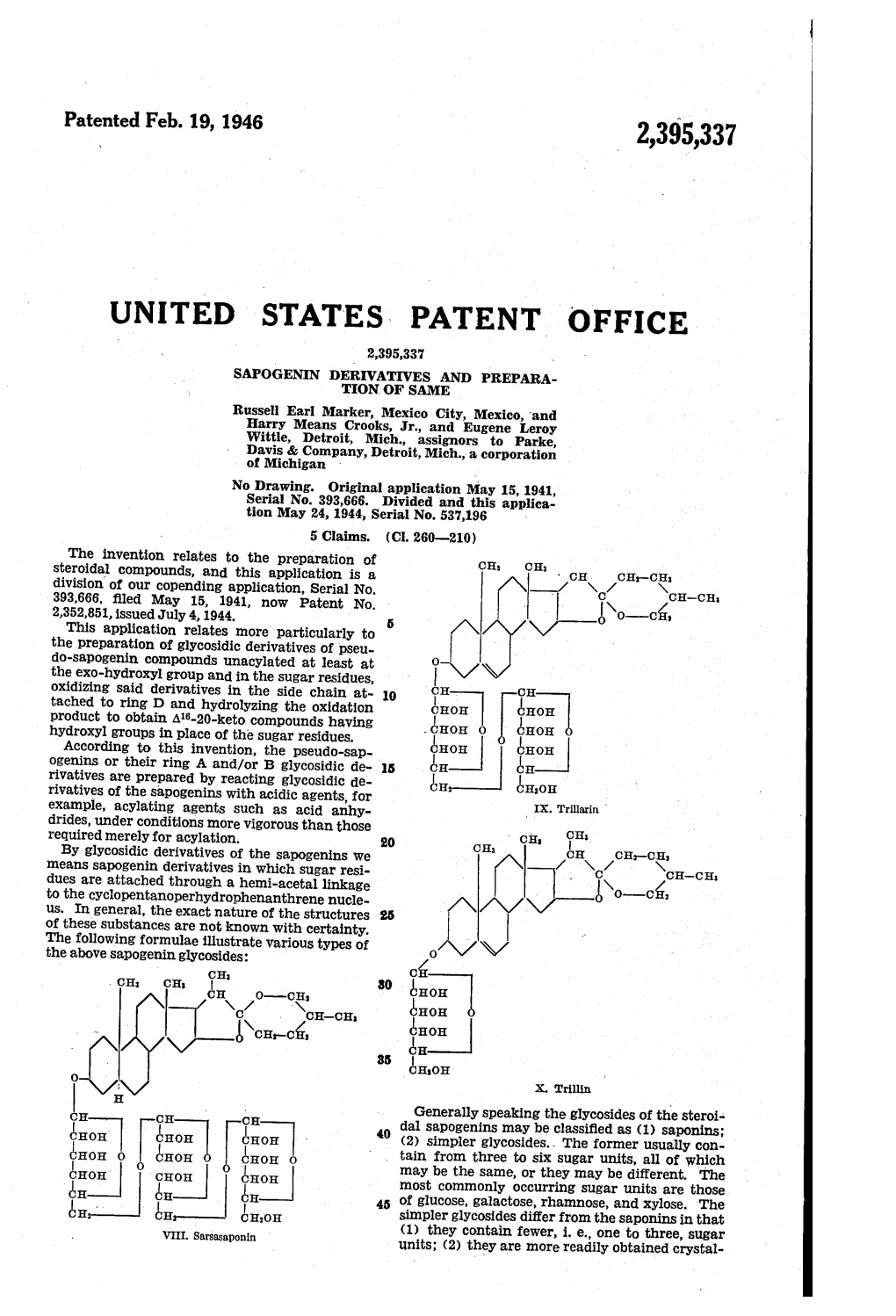
El E. in Simpler Glycosides Differ from the Saponins in That VIII
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Saponins from Chinese Medicines As Anticancer Agents
molecules Review Saponins from Chinese Medicines as Anticancer Agents Xiao-Huang Xu 1,†, Ting Li 1,†, Chi Man Vivienne Fong 1, Xiuping Chen 1, Xiao-Jia Chen 1, Yi-Tao Wang 1, Ming-Qing Huang 2,* and Jin-Jian Lu 1,* 1 State Key Laboratory of Quality Research in Chinese Medicine, Institute of Chinese Medical Sciences, University of Macau, Macao, China; [email protected] (X.-H.X.); [email protected] (T.L.); [email protected] (C.M.V.F.); [email protected] (X.C.); [email protected] (X.-J.C.); [email protected] (Y.-T.W.) 2 College of Pharmacy, Fujian University of Traditional Chinese Medicine, Fuzhou 350122, China * Correspondence: [email protected] (M.-Q.H.); [email protected] (J.-J.L.); Tel.: +86-591-2286-1135 (M.-Q.H.); +85-388-224-674 (J.-J.L.) † The authors contribute equally to this work. Academic Editor: Derek J. McPhee Received: 15 August 2016; Accepted: 30 September 2016; Published: 5 October 2016 Abstract: Saponins are glycosides with triterpenoid or spirostane aglycones that demonstrate various pharmacological effects against mammalian diseases. To promote the research and development of anticancer agents from saponins, this review focuses on the anticancer properties of several typical naturally derived triterpenoid saponins (ginsenosides and saikosaponins) and steroid saponins (dioscin, polyphyllin, and timosaponin) isolated from Chinese medicines. These saponins exhibit in vitro and in vivo anticancer effects, such as anti-proliferation, anti-metastasis, anti-angiogenesis, anti-multidrug resistance, and autophagy regulation actions. In addition, related signaling pathways and target proteins involved in the anticancer effects of saponins are also summarized in this work. -

In Chemistry, Glycosides Are Certain Molecules in Which a Sugar Part Is
GLYCOSIDES Glycosides may be defined as the organic compounds from plants or animal sources, which on enzymatic or acid hydrolysis give one or more sugar moieties along with non- sugar moiety. Glycosides play numerous important roles in living organisms. Many plants store important chemicals in the form of inactive glycosides; if these chemicals are needed, the glycosides are brought in contact with water and an enzyme, and the sugar part is broken off, making the chemical available for use. Many such plant glycosides are used as medications. In animals (including humans), poisons are often bound to sugar molecules in order to remove them from the body. Formally, a glycoside is any molecule in which a sugar group is bonded through its carbon atom to another group via an O-glycosidic bond or an S-glycosidic bond; glycosides involving the latter are also called thioglycosides. The sugar group is then known as the glycone and the non-sugar group as the aglycone or genin part of the glycoside. The glycone can consist of a single sugar group (monosaccharide) or several sugar groups (oligosaccharide). Classification Classification based on linkages Based on the linkage of sugar moiety to aglycone part 1. O-Glycoside:-Here the sugar is combined with alcoholic or phenolic hydroxyl function of aglycone.eg:-digitalis. 2. N-glycosides:-Here nitrogen of amino group is condensed with a sugar ,eg- Nucleoside 3. S-glycoside:-Here sugar is combined with sulphur of aglycone,eg- isothiocyanate glycosides. 4. C-glycosides:-By condensation of a sugar with a cabon atom, eg-Cascaroside, aloin. Glycosides can be classified by the glycone, by the type of glycosidic bond, and by the aglycone. -

Traditional Medicine Research Doi: 10.12032/TMR20210616237
Traditional Medicine Research doi: 10.12032/TMR20210616237 Annual advances of integrative pharmacology in 2020 Ke-Wu Zeng1*, Ming-Yao Gu2* 1State Key Laboratory of Natural and Biomimetic Drugs, School of Pharmaceutical Sciences, Peking University, Beijing 100191, China; 2Department of Cell Biology and Medical Genetics, School of Basic Medical Sciences, Shenzhen University Health Science Center, Shenzhen 518061, China. *Corresponding to: Ke-Wu Zeng, State Key Laboratory of Natural and Biomimetic Drugs, School of Pharmaceutical Sciences, Peking University, No.38 Xueyuan Road, Haidian District, Beijing 100191, China; E-mail: [email protected]. Ming-Yao Gu, Department of Cell Biology and Medical Genetics, School of Basic Medical Sciences, Shenzhen University Health Science Center, No.1066 Xueyuan Avenue, Nanshan District, Shenzhen 518061, China; E-mail: [email protected]. Highlights This review covers the studies in the year 2020 for pharmacological reports on traditional medicine as well as herb-derived active natural products. Moreover, the pharmacological reports on active natural products against cancers, inflammation, and metabolic diseases were major topics. Tradition This annual integrative pharmacology review includes the reports published in 2020 on bioactive herbal extracts and novel compounds in traditional medicine. Pharmacological reports on traditional herbs as well as their active compounds for anticancer, inflammation, and metabolic diseases occupy dominant positions. Submit a manuscript: https://www.tmrjournals.com/tmr 1 doi: 10.12032/TMR20210616237 REVIEW Abstract Major studies on the pharmacology of traditional herbs as well as active compounds have been introduced in this review over the previous 12 months. This annual integrative pharmacology review includes the reports published in 2020 on bioactive herbal extracts and novel compounds in traditional medicine. -

Print This Article
PEER-REVIEWED ARTICLE bioresources.com GC-MS Characterisation of Sapogenins from Sisal Waste and a Method to Isolate Pure Hecogenin Jener David G. Santos * and Alexsandro Branco ** Five steroidal sapogenins (tigogenin, neotigogenina, hecogenin, gloriogenin, and dehydrohecogenin) were characterised by gas chromatography coupled with mass spectrometry (GC-MS) from a hydrolysed extract of sisal waste. In addition, pure hecogenin, an important raw material for the pharmaceutical industry, was obtained from this waste by selective liquid-liquid extraction of saponins with only hecogenin as aglycone, followed by acid hydrolysis. The yield of pure hecogenin was 460 mg.Kg-1 of sisal waste. Keywords: Agave sisalana; Sisal waste; Extraction; Steroids; Hecogenin Contact information: Laboratory of Phytochemistry, State University of Feira de Santana, 44.036-900 Feira de Santana, Bahia, Brazil; Corresponding authors: *[email protected], **[email protected] INTRODUCTION Steroidal sapogenins are a glycone non-sugar portion of the saponin molecule used for the semi-synthesis of bioactive compounds. Example compounds used in this application include the following: smilagenin, sarsasapogenin, diosgenin, yamogenin, tigogenin, neotigogenin, gloriogenin, gentrogenin, hecogenin, sisalagenin, 9-dehydro- hecogenin, and gitogenin (Agrawal et al. 1985). Among these steroidal sapogenins, diosgenin, sarsasapogenin, and hecogenin are particularly important. The usefulness of hecogenin (Fig. 1) as a synthetic starting material is due to the presence of an oxygen atom in the C-12 position that can be moved to the C-11 position. This makes it possible to introduce the 9-11 double bond required for the syntheses of corticosteroids (Beauvoir 1976). Fig. 1. Chemical structural of hecogenin In the 1940s, steroidal sapogenins achieved great economic importance because of their transformation into pharmaceutically valuable derivatives such as corticosteroids (prednisone, dexamethasone, betamethasone, triamcinolone, and others), sexual hormones, and steroid diuretics (Fernández-Herrera et al. -

United States Patent Office W
w 2,870,143 United States Patent Office Patented Jan. 20, 1959 2 2,870,143 the structure of the parent sapogenin and that heating beyond the point of complete conversion gives lower PROCESS FOR CONVERSION OF STEROIDAL yields of the pseudosapogenins. In particular, differ SAPOGENINS TOPSEUDOSAPOGENNS ences in the spiroketal side chain configuration of Sapog Monroe E. Wall, Oreland, and Samuel Serota, Phila eninsis one factor which leads to significant differences delphia, Pa., assignors to the United States of America in the rate of conversion of the sapogenin to pseudo as represented by the Secretary of Agriculture sapogenin. In the accompanying table (Table I) each No Drawing. Application January 22, 1958 pair of sapogenins is identical except for isomerism in the spiroketal side chain. The site of isomerism, now Serial No. 710,588 10 considered to be at C25 (cf. M. E. Wall, Experientia 11, i8. Claims. (Cl. 260-239.55) 340 (1955), for a review of pertinent literature), is im material to the present invention. The significance of (Granted under Title 35, U.S. Code (1952), sec. 266) the data in Table I is that in each pair of normal and A non-exclusive, irrevocable, royalty-free license in the iso sapogenins, the normal isomer is converted to its re invention herein described, throughout the world for all 5 spective pseudosapogenin much more rapidly than is the purposes of the United States Government, with the power iso analogue. to grant sublicenses for such purposes, is hereby granted TABLE. I.-CONVERSION OF NATURAL SAPOGENINS TO to the Government of the United States of America. -

International Historic Chemical Landmark Acclaims Success of Mexican Steroid Industry and a U.S
Journal of the Mexican Chemical Society ISSN: 1870-249X [email protected] Sociedad Química de México México Raber, Linda Steroid industry honored. International historic chemical landmark acclaims success of mexican steroid industry and a U.S. chemist who made it possible Journal of the Mexican Chemical Society, vol. 43, núm. 6, noviembre-diciembre, 1999, pp. 235-237 Sociedad Química de México Distrito Federal, México Available in: http://www.redalyc.org/articulo.oa?id=47543610 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative Revista de la Sociedad Química de México, Vol. 43, Núm. 6 (1999) 235-237 Noticias Steroid Industry Honored† International Historic Chemical Landmark Acclaims Success of Mexican Steroid Industry and a U.S. Chemist Who Made it Possible Linda Raber American Chemical Society 1155-16th St., N.W, Washington, D.C. 20036. U.S.A. “There are more stories told about Russell Marker than any he founded in Mexico City with Emeric Somlo and Federico other chemist. Although perhaps many of these stories are A. Lehmann. apocryphal, they are so fascinating that most of us cannot bear “This low-cost progesterone eventually became the pre- to stop repeating them. This is the oral history of our profes- ferred precursor in the industrial preparation of the anti- sion that we pass to our colleagues and our students. They are inflammatory drug cortisone. In 1951, Syntex researchers syn- the campfire stories that bind our profession together” – thesized the first useful oral contraceptive from Marker’s start- Steven M. -

Anticancer Properties of Phytochemicals Present in Medicinal Plants of North America
Chapter 6 Anticancer Properties of Phytochemicals Present in Medicinal Plants of North America Wasundara Fernando and H. P. Vasantha Rupasinghe Additional information is available at the end of the chapter http://dx.doi.org/10.5772/55859 1. Introduction Cancer is one of the most severe health problems in both developing and developed countries, worldwide. Among the most common (lung, stomach, colorectal, liver, breast) types of cancers, lung cancer has continued to be the most common cancer diagnosed in men and breast cancer is the most common cancer diagnosed in women. An estimated 12.7 million people were diagnosed with cancer across the world in 2008, and 7.6 million people died from the cancer during the same year [1]. Lung cancer, breast cancer, colorectal cancer and stomach cancer accounted for two-fifths of the total cases of cancers diagnosed worldwide [1]. More than 70% of all cancer deaths occurred in low- and middle-income countries. Deaths due to cancer are projected to continuously increase and it has been estimated that there will be 11.5 million deaths in the year 2030 [1] and 27 million new cancer cases and 17.5 million cancer deaths are projected to occur in the world by 2050 [2]. According to Canadian cancer statistics, issued by the Canadian Cancer Society, it is estimated that 186,400 new cases of cancer (excluding 81,300 non-melanoma skin cancers) and 75,700 deaths from cancer will occur in Canada in 2012 [1]. The lowest number of incidences and mortality rate is recorded in British Columbia. Both incidence and mortality rates are higher in Atlantic Canada and Quebec [3]. -

RP-HPLC Analysis of Derivatized Sapogenin of Asparagus)
Journal of Medicinal Plants Research Vol. 5(10), pp. 1900-1904, 18 May, 2011 Available online at http://www.academicjournals.org/JMPR ISSN 1996-0875 ©2011 Academic Journals Full Length Research Paper High performance liquid chromatographic analysis of derivatized sapogenin of Asparagus (RP-HPLC analysis of derivatized sapogenin of Asparagus) Jagmohan S. Negi 1*, Pramod Singh 2, Geeta Joshi nee Pant 2 and M. S. M. Rawat 2 1Herbal Research and Development Institute, Mandal-Gopeshwar (Chamoli)-246401, Uttarakhand, India 2Department of Chemistry, HNB Garhwal University, Srinagar (Garhwal)-246 174, Uttarakhand, India. Accepted 28 September, 2010 RP-HPLC method on C 8 column was investigated for derivatized sapogenin, the marker in Asparagus species using water and acetonitrile as mobile phase in isocratic manner at a flow rate of 0.5 ml/min. The method is sensitive (LOD = 2.02 µg), accurate (average recovery was 98.97%) and precise (intra day variation of RT<0.398, inter day variation of RT<0.982). Quantification was done by UV absorption at 210 nm. Rhizome has maximum concentration of bioactive ranging in between 0.10 to 3.72. Key words: Asparagus racemosus, A. curillus , RP-HPLC, sarsasapogenin, acetyl sarsasapogenin, quantification. INTRODUCTION Asparagus racemosus and A. curillus (family liliacae) Sarsasapogenin, a saturated steroidal genin does not commonly known as Shatavari or Jhirna are wild growing absorb in the λ range 200 to 210 nm, hence this is the first shrubs cultivated in some nurseries of India. These are report of HPLC study of variation of sarsasaoigenin as its distributed in the tropical and subtropical regions and are acetyl derivative in A. -

The Birth of the Pill
The contraceptive pill The birth of the pill Most primitive societies knew of plants that would control their fertility, but until 1960 there was no clinically proven drug that provided a reliable method of contraception. Fifty years on, John Mann reports on the conception and evolution of the contraceptive pill In Mexico City in 1943, the small the same basic ring system as carry out similar chemistry on the company Laboratorios Hormona In short cholesterol but with an oxidised more accessible diosgenin from the occupied a niche in the market The first cheap and side-chain. He was intrigued by the genus Dioscorea. for pharmaceuticals. Its founders, simple route to make possibility of using these oxidised Being something of a botanist as Hungarian Emeric Somlo and progesterone, a key sites to affect a cleavage of the well as a chemist, Marker headed German Federico Lehmann, were hormone in the pill, was side-chain and make the degraded across the border into Mexico in making a modest living selling developed in the 1940s structures of the sex hormones. search of Dioscorea mexicana – the natural hormones extracted from The first pill was This is, of course, exactly what Mexican yam that the locals called animal organs. launched in 1960, and by happens in the enzyme-mediated cabeza de negro. After considerable Imagine their surprise when one 1966 more than 5 million oxidative metabolism of cholesterol difficulties he found a good source day a rather eccentric American US women were using to produce progesterone (see near the city of Orizaba in Veracruz professor of chemistry called Russell oral contraceptives Chemistry World, November 2009, State, and transported large Marker arrived in their office There have been no p54). -

Saponins As Cytotoxic Agents: a Review
Phytochem Rev (2010) 9:425–474 DOI 10.1007/s11101-010-9183-z Saponins as cytotoxic agents: a review Irma Podolak • Agnieszka Galanty • Danuta Sobolewska Received: 13 January 2010 / Accepted: 29 April 2010 / Published online: 25 June 2010 Ó The Author(s) 2010. This article is published with open access at Springerlink.com Abstract Saponins are natural glycosides which Con A Concanavalin A possess a wide range of pharmacological properties ER Endoplasmic reticulum including cytotoxic activity. In this review, the recent ERK Extracellular signal-regulated studies (2005–2009) concerning the cytotoxic activity kinase of saponins have been summarized. The correlations GADD Growth arrest and DNA damage- between the structure and the cytotoxicity of both inducible gene steroid and triterpenoid saponins have been described GRP Glucose regulated protein as well as the most common mechanisms of action. hTERT Telomerase reverse transcriptase JAK Janus kinase Keywords Cytotoxic mechanisms Á MEK = MAPK Mitogen-activated protein kinase Glycosides Á Sar Á Steroid Á Triterpenoid MMP Matrix metalloproteinase mTOR Mammalian target of rapamycin Abbreviations NFjB Nuclear factor kappa-light-chain- AMPK AMP activated protein kinase enhancer of activated B cells BiP Binding protein NO Nitric oxide BrDU Bromodeoxyuridine PARP Poly ADP ribose polymerase CCAAT Cytidine-cytidine-adenosine- PCNA Proliferating cell nuclear antigen adenosine-thymidine PI3K Phosphoinositide-3-kinase CD Cluster of differentiation molecule PP Protein phosphatase CDK Cyclin-dependent kinase PPAR-c Peroxisome proliferator-activated CEBP CCAAT-enhancer-binding protein receptor c CHOP CEPB homology protein Raf Serine/threonine specific kinase STAT Signal transducer and activator of transcription TIMP Tissue inhibitor of metallo- proteinase TSC Tuberous sclerosis complex VEGF Vascular endothelial growth factor & I. -

Ben L. Feringa University of Groningen
In November 1941, while paging through a botany text, he saw a promising picture of dioscorea, a type of wild The Marker Lectures honor yam that grew in the Mexican state of Vera Cruz near Professor Russell Marker, a Orizaba. Marker went to Mexico, collected two big University of Maryland Hall of roots of dioscorea, loaded them into bags, and put them Fame alumnus and on the top of a bus. When he got to Orizaba, the bags extraordinarily inventive were gone, but he recovered the larger, 50-lb root by organic chemist. Marker, who bribing a policeman. The tubar, which he ended up was born on his father’s farm smuggling out of the country, yielded a good quantity near Hagerstown, MD, in 1902, of diosgenin—a convenient and cheap starting material received a bachelor’s degree that he believed could yield progesterone by the ton. from the University of Marker’s discovery was about to change progesterone Maryland in 1923 and a from a costly rarity to the cheapest of all steroid hormones. In a 1979 interview with Stanford master’s degree. in physical University chemist Carl Djerassi, another pioneer of the chemistry in 1924. He then steroid hormone industry, Marker recalled that he could started doctoral research with Morris Kharasch at not convince Parke-Davis to support the Maryland. Within a year, Marker had completed enough commercialization of his synthesis. So Marker work for his thesis but still needed to take some physical withdrew all of his savings from the bank, went down chemistry courses. Marker considered this physical to Vera Cruz, and collected 9 or 10 tons of the roots, chemistry requirement a waste of time and refused to extracted the root with alcohol and evaporated it down take them because he had taken the courses as an to syrup that he took back to the U.S. -

United States Patent Office Patented Oct
2,719,845 United States Patent Office Patented Oct. 4, 1955 2 Example I 2,719,845 5 kg. of dried yucca leaf meal were extracted with 25 1. of boiling 80% isopropanol-20% water. The solution ISOLATION OF SAPOGENINS was cooled, filtered and concentrated to 41. The con Monroe E. Wall, Oreland, Pa., assignor to the United centrate was heated to boiling and filtered. The fatty States of America as represented by the Secretary of residue was washed with hot 50% isopropanol-50% Agriculture water. The combined filtrates were concentrated to 4 1. No Drawing. Application December 2, 1952, and the essentially aqueous liquor was extracted 4 times Serial No. 323,735 by shaking with 1 i. of butanol each time. To the com 10 bined butanol extracts 21. of water was added. The mix 3 Claims. (C. 260-239.55) ture was then reduced to 1 l. by distilling off the butanol water azeotrope. The residue consisted of an essentially (Granted under Title 35, U.S. Code (1952), sec. 266) aqueous solution of crude steroidal saponins. The steroidal saponins were hydrolyzed by adding 250 A non-exclusive, irrevocable, royalty-free license in the 5 ml. of ethanol and sufficient hydrochloric acid to make invention herein described for all governmental purposes, the solution 2-normal in acid. The acidic solution was throughout the world, with power to grant sub-licenses for refluxed 3 hours, cooled and filtered. The crude steroidal Such purposes, is hereby granted to the Government of sapogenin thus obtained was then purified by the process the United States of America.