Albatross-Borne Loggers Show Feeding on Deep-Sea Squids: Implications for the Study of Squid Distributions
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Forage Fish Management Plan
Oregon Forage Fish Management Plan November 19, 2016 Oregon Department of Fish and Wildlife Marine Resources Program 2040 SE Marine Science Drive Newport, OR 97365 (541) 867-4741 http://www.dfw.state.or.us/MRP/ Oregon Department of Fish & Wildlife 1 Table of Contents Executive Summary ....................................................................................................................................... 4 Introduction .................................................................................................................................................. 6 Purpose and Need ..................................................................................................................................... 6 Federal action to protect Forage Fish (2016)............................................................................................ 7 The Oregon Marine Fisheries Management Plan Framework .................................................................. 7 Relationship to Other State Policies ......................................................................................................... 7 Public Process Developing this Plan .......................................................................................................... 8 How this Document is Organized .............................................................................................................. 8 A. Resource Analysis .................................................................................................................................... -

Measurements and Regressions of Otoliths, Cephalopod Beaks, and Other Prey Hard Parts Used to Reconstruct California Current Predator Diet Composition
NOAA Technical Memorandum NMFS DECEMBER 2020 MEASUREMENTS AND REGRESSIONS OF OTOLITHS, CEPHALOPOD BEAKS, AND OTHER PREY HARD PARTS USED TO RECONSTRUCT CALIFORNIA CURRENT PREDATOR DIET COMPOSITION 1Mark S. Lowry, 1K. Alexandra Curtis, 2Christiana M. Boerger 1Southwest Fisheries Science Center National Marine Fisheries Service National Oceanic and Atmospheric Administration 8901 La Jolla Shores Dr. San Diego, CA 92037 2California State University Northridge Northridge, CA NOAA-TM-NMFS-SWFSC-637 U.S. DEPARTMENT OF COMMERCE National Oceanic and Atmospheric Administration National Marine Fisheries Service Southwest Fisheries Science Center About the NOAA Technical Memorandum series The National Oceanic and Atmospheric Administration (NOAA), organized in 1970, has evolved into an agency which establishes national policies and manages and conserves our oceanic, coastal, and atmospheric resources. An organizational element within NOAA, the Office of Fisheries is responsible for fisheries policy and the direction of the National Marine Fisheries Service (NMFS). In addition to its formal publications, the NMFS uses the NOAA Technical Memorandum series to issue informal scientific and technical publications when complete formal review and editorial processing are not appropriate or feasible. Documents within this series, however, reflect sound professional work and may be referenced in the formal scientific and technical literature. SWFSC Technical Memorandums are available online at the following websites: SWFSC: https://www.fisheries.noaa.gov/about/southwest-fisheries-science-center NOAA Repository: https://repository.library.noaa.gov/ NTIS National Technical Reports Library: https://ntrl.ntis.gov/NTRL/ Accessibility information NOAA Fisheries Southwest Fisheries Science Center (SWFSC) is committed to making our publications and supporting electronic documents accessible to individuals of all abilities. -
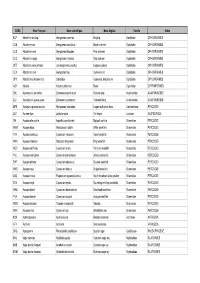
Nom Français
CODE Nom Français Nom scientifique Nom Anglais Famille Ordre KCP Abadèche du Cap Genypterus capensis Kingklip Ophidiidae OPHIDIIFORMES CUB Abadèche noir Genypterus maculatus Black cusk-eel Ophidiidae OPHIDIIFORMES CUS Abadèche rosé Genypterus blacodes Pink cusk-eel Ophidiidae OPHIDIIFORMES CUC Abadèche rouge Genypterus chilensis Red cusk-eel Ophidiidae OPHIDIIFORMES OFZ Abadèche sans jambes Lamprogrammus exutus Legless cuskeel Ophidiidae OPHIDIIFORMES CEX Abadèches nca Genypterus spp Cusk-eels nei Ophidiidae OPHIDIIFORMES OPH Abadèches, brotules nca Ophidiidae Cusk-eels, brotulas nei Ophidiidae OPHIDIIFORMES ALR Ablette Alburnus alburnus Bleak Cyprinidae CYPRINIFORMES ZML Acanthure à pierreries Zebrasoma gemmatum Spotted tang Acanthuridae ACANTHUROIDEI ZLV Acanthure à queue jaune Zebrasoma xanthurum Yellowtail tang Acanthuridae ACANTHUROIDEI MPS Achigan à grande bouche Micropterus salmoides Largemouth black bass Centrarchidae PERCOIDEI LQT Acmée râpe Lottia limatula File limpet Lottiidae GASTROPODA ISA Acoupa aile-courte Isopisthus parvipinnis Bigtooth corvina Sciaenidae PERCOIDEI WEW Acoupa blanc Atractoscion nobilis White weakfish Sciaenidae PERCOIDEI YNV Acoupa cambucu Cynoscion virescens Green weakfish Sciaenidae PERCOIDEI WKK Acoupa chasseur Macrodon ancylodon King weakfish Sciaenidae PERCOIDEI WEP Acoupa du Pérou Cynoscion analis Peruvian weakfish Sciaenidae PERCOIDEI YNJ Acoupa mongolare Cynoscion jamaicensis Jamaica weakfish Sciaenidae PERCOIDEI SWF Acoupa pintade Cynoscion nebulosus Spotted weakfish Sciaenidae PERCOIDEI WKS Acoupa -

Common Sea Life of Southeastern Alaska a Field Guide by Aaron Baldwin & Paul Norwood
Common Sea Life of Southeastern Alaska A field guide by Aaron Baldwin & Paul Norwood All pictures taken by Aaron Baldwin Last update 08/15/2015 unless otherwise noted. [email protected] Table of Contents Introduction ….............................................................…...2 Acknowledgements Exploring SE Beaches …………………………….….. …...3 It would be next to impossible to thanks everyone who has helped with Sponges ………………………………………….…….. …...4 this project. Probably the single-most important contribution that has been made comes from the people who have encouraged it along throughout Cnidarians (Jellyfish, hydroids, corals, the process. That is why new editions keep being completed! sea pens, and sea anemones) ……..........................…....8 First and foremost I want to thanks Rich Mattson of the DIPAC Macaulay Flatworms ………………………….………………….. …..21 salmon hatchery. He has made this project possible through assistance in obtaining specimens for photographs and for offering encouragement from Parasitic worms …………………………………………….22 the very beginning. Dr. David Cowles of Walla Walla University has Nemertea (Ribbon worms) ………………….………... ….23 generously donated many photos to this project. Dr. William Bechtol read Annelid (Segmented worms) …………………………. ….25 through the previous version of this, and made several important suggestions that have vastly improved this book. Dr. Robert Armstrong Mollusks ………………………………..………………. ….38 hosts the most recent edition on his website so it would be available to a Polyplacophora (Chitons) ……………………. -

Onykia and Identification of Their Paralarvae from Northern Hawaiian Waters
J. Mar. Biol. Ass. U.K. (2007), 87, 959–965 doi: 10.1017/S0025315407056196 Printed in the United Kingdom Molecular evidence for synonymy of the genera Moroteuthis and Onykia and identification of their paralarvae from northern Hawaiian waters Toshie Wakabayashi*, Tsunemi Kubodera†, Mitsuo Sakai*, Taro Ichii* and Seinen Chow‡∫ *National Research Institute of Far Seas Fisheries, 2-12-4 Fukuura, Kanazawa-ku, Yokohama, Kanagawa 236-8648, Japan. †National Science Museum, Tokyo, 3-23-1 Hyakunin-cho, Shinjuku-ku, Tokyo 169-0073, Japan. ‡National Research Institute of Fisheries Science, 6-31-1 Nagai, Yokosuka, Kanagawa 283-0316, Japan. ∫Corresponding author, e-mail: [email protected] It has been claimed that most squid species in the genus Onykia may be immature stages of species in the genus Moroteuthis. To evaluate the generic status of Moroteuthis and Onykia and to identify paralarvae collected in northern Hawaiian waters, we performed morphological investigation and nucleotide sequence analysis of the mitochondrial cytochrome oxidase I (COI) gene. Of 42 Onykia paralarvae (1.8–8.5 mm dorsal mantle length, DML) examined, 41 had a nucleotide sequence identical to that of M. robusta and one (designated Onykia sp. A) could not be assigned to any known Moroteuthis species. Nucleotide sequence diversity estimates based on Kimura’s two-parameter distances between Onykia sp. A and Moroteuthis spp. (0.109–0.150) fell well within the range of congeneric species, suggesting that Onykia sp. A is a member of the genus Moroteuthis. Molecular data supported the conclusion that the genus Moroteuthis is a junior synonym of the genus Onykia. -

Oregon Forage Fish Management Plan
Oregon Forage Fish Management Plan June 15, 2016 DRAFT Oregon Department of Fish and Wildlife Marine Resources Program 2040 SE Marine Science Drive Newport, OR 97365 (541) 867-4741 http://www.dfw.state.or.us/MRP/ Oregon Department of Fish & Wildlife · DRAFT · 1 Table of Contents Executive Summary ....................................................................................................................................... 4 Introduction .................................................................................................................................................. 6 Purpose and Need ..................................................................................................................................... 6 Federal action to protect Forage Fish (2016)............................................................................................ 7 The Oregon Marine Fisheries Management Plan Framework .................................................................. 7 Relationship with Other State Policies ...................................................................................................... 7 How this Document is Organized .............................................................................................................. 8 A. Resource Analysis ..................................................................................................................................... 9 A.1. Description of the species included in the Plan ................................................................................ -

Systematics of the Onychoteuthidae Gray, 1847 (Cephalopoda: Oegopsida)
Zootaxa 2696: 1–186 (2010) ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ Monograph ZOOTAXA Copyright © 2010 · Magnolia Press ISSN 1175-5334 (online edition) ZOOTAXA 2696 Systematics of the Onychoteuthidae Gray, 1847 (Cephalopoda: Oegopsida) KATHRIN S. R. BOLSTAD Earth & Oceanic Sciences Research Institute, Auckland University of Technology, Private Bag 92006, Auckland 1142, New Zealand [email protected] Magnolia Press Auckland, New Zealand Accepted by D. Geiger: 12 Nov. 2010; published: 3 Dec. 2010 KATHRIN S. R. BOLSTAD Systematics of the Onychoteuthidae Gray, 1847 (Cephalopoda: Oegopsida) (Zootaxa 2696) 186 pp.; 30 cm. 3 December 2010 ISBN 978-1-86977-627-5 (paperback) ISBN 978-1-86977-628-2 (Online edition) FIRST PUBLISHED IN 2010 BY Magnolia Press P.O. Box 41-383 Auckland 1346 New Zealand e-mail: [email protected] http://www.mapress.com/zootaxa/ © 2010 Magnolia Press All rights reserved. No part of this publication may be reproduced, stored, transmitted or disseminated, in any form, or by any means, without prior written permission from the publisher, to whom all requests to reproduce copyright material should be directed in writing. This authorization does not extend to any other kind of copying, by any means, in any form, and for any purpose other than private research use. ISSN 1175-5326 (Print edition) ISSN 1175-5334 (Online edition) 2 · Zootaxa 2696 © 2010 Magnolia Press BOLSTAD Table of contents ABSTRACT . 4 INTRODUCTION . 4 MATERIALS & METHODS . 7 SYSTEMATICS . 14 Family Onychoteuthidae Gray, 1847 . 14 Onychoteuthis Lichtenstein, 1818 . 17 Onychoteuthis banksii (Leach, 1817) . 18 Onychoteuthis bergii Lichtenstein, 1818 . 23 Onychoteuthis aequimanus Gabb, 1868 . -

The Giant Deep-Sea Octopus Haliphron Atlanticus Forages on Gelatinous Fauna Received: 31 October 2016 H.J.T
www.nature.com/scientificreports OPEN The giant deep-sea octopus Haliphron atlanticus forages on gelatinous fauna Received: 31 October 2016 H.J.T. Hoving1 & S.H.D. Haddock2 Accepted: 16 February 2017 Feeding strategies and predator-prey interactions of many deep-sea pelagic organisms are still Published: 27 March 2017 unknown. This is also true for pelagic cephalopods, some of which are very abundant in oceanic ecosystems and which are known for their elaborate behaviors and central role in many foodwebs. We report on the first observations of the giant deep-sea octopusHaliphron atlanticus with prey. Using remotely operated vehicles, we saw these giant octopods holding medusae in their arms. One of the medusae could be identified asPhacellophora camtschatica (the egg-yolk jelly). Stomach content analysis confirmed predation on cnidarians and gelatinous organisms. The relationship between medusae and H. atlanticus is discussed, also in comparison with other species of the Argonautoidea, all of which have close relationships with gelatinous zooplankton. The pelagic ocean is the largest living space on the planet, and one that holds enormous biodiversity and biomass1. While general trophic relations between oceanic pelagic organisms can be reconstructed using various dietary tracers2, knowledge of prey choice and feeding behavior remains virtually unknown for many pelagic fauna. With the advancement of underwater technology, deep-sea in situ observations are revealing novel behaviors of deep-sea organisms. Examples include luring in deep-sea siphonophores3 and squids4,5, carnivory and prey specialization in ctenophores6 and medusae7, and detritivory in cephalopods8. Cephalopods are a group of molluscs that inhabit the marine environment from shallow reefs to the deep sea. -

Graphical Abstract
Graphical Abstract Highlights Our results support monophyly of the family Onychoteuthidae and each of its seven genera Total onychoteuthid species diversity likely exceeds 34 species, making it the third-most diverse oegopsid (deep-sea squid) family 25% (n = 7) of the species recovered in the present study appear to represent undescribed taxa The genus name ‘Kondakovia’ is recognized as a junior synonym of Moroteuthopsis, which presently contains three species including the abundant and ecologically important Southern Hemisphere species M. ingens A mitochondrial phylogeny of the family Onychoteuthidae Gray, 1847 (Cephalopoda: Oegopsida) Bolstad, Kat SRa; Braid, Heather Ea; Strugnell, Jan Mb; Lindgren, Annie R c; Lischka, Alexandraa; Kubodera, Tsunemid; Laptikhovsky, Vlad Le; Roura Labiaga, Alvarof (a) AUT Lab for Cephalopod Ecology & Systematics, School of Science, Auckland University of Technology, Auckland, New Zealand [email protected] (b) Centre for Sustainable Tropical Fisheries and Aquaculture, James Cook University, Townsville, Queensland 4810, Australia (c) Center for Life in Extreme Environments, Department of Biology, Portland State University, Portland, Oregon, USA (d) National Museum of Nature and Science, Curator Emeritus, 4-1-1 Amakubo, Tsukuba, Ibaraki 305- 0005, Japan (e) CEFAS, Pakefield Rd., Lowestoft NR330HT, United Kingdom (f) Instituto de Investigaciones Marinas de Vigo (IIM-CSIC), Vigo, Spain Abstract The oegopsid squid family Onychoteuthidae was recently revised based on morphology, but sufficient material -

Systematics of the Onychoteuthidae Gray, 1847 (Cephalopoda: Oegopsida)
Systematics of the Onychoteuthidae Gray, 1847 (Cephalopoda: Oegopsida) K.S. Bolstad A thesis submitted to the Earth & Oceanic Sciences Research Institute Auckland University of Technology in fulfilment of the requirements for the degree of Doctor of Philosophy supervised by Dr Steve O’Shea 2008 i TABLE OF CONTENTS List of Tables............................................................................................................... iii List of Figures..............................................................................................................iv Abstract....................................................................................................................... 1 Introduction ................................................................................................................. 3 Foreword — Generic and Familial Nomenclature....................................................... 3 Introduction ................................................................................................................. 4 Materials & Methods ................................................................................................. 13 Checklist of Species.................................................................................................. 24 Quick Visual Reference to the Onychoteuthidae ...................................................... 25 Systematics............................................................................................................... 31 Onychoteuthis ...................................................................................................... -

Nishizawa-Et-Al.-2018-MEPS.Pdf
Vol. 592: 257–265, 2018 MARINE ECOLOGY PROGRESS SERIES Published March 29 https://doi.org/10.3354/meps12482 Mar Ecol Prog Ser Albatross-borne loggers show feeding on deep-sea squids: implications for the study of squid distributions Bungo Nishizawa1,*, Takanori Sugawara2, Lindsay C. Young3, Eric A. Vanderwerf 3, Ken Yoda2, Yutaka Watanuki1 1Graduate School of Fisheries Sciences, Hokkaido University, 3-1-1, Minato, Hakodate, Hokkaido 041-8611, Japan 2Graduate School of Environmental Studies, Nagoya University, Furo, Chikusa, Nagoya, Japan 3Pacific Rim Conservation, 3038 Oahu Avenue, Honolulu, HI 96822, USA ABSTRACT: How surface-feeding albatrosses feed on deep-sea squids has long been a mystery. We investigated foraging behavior during daylight hours of 20 Laysan albatrosses Phoebastria immutabilis breeding in Hawaii using GPS- and camera-loggers. The birds traveled to the North Pacific Transition Zone up to 600 km north of their breeding site. The camera images showed that Laysan albatrosses fed on large (~1 m body length), intact floating dead squids (6 events) and floating fragmented squids (10 events) over deep oceanic water (>2000 m) while they flew in a straight path without sinuous searching. Feeding events on squids were not observed during trips when fishing vessels were photographed and seemed to be distributed randomly and sparsely. Thus, this study suggests that Laysan albatrosses found large, presumably post-spawning, squids opportunistically while they were traveling during daylight hours. Although we did not find cetaceans in our surface pictures, we could not rule out the possibility that birds fed on squids, especially fragmented specimens, in the regurgitates of cetaceans at depth. -

A Review of the Deep-Sea Cephalopod Fauna Off the Pacific
Deep-sea Fauna and Pollutants off Pacifi c Coast of Northern Japan, edited by T. Fujita, National Museum of Nature and Science Monographs, No. 39, pp. 385-404, 2009 A Review of the Deep-sea Cephalopod Fauna off the Pacifi c Coast of Northeastern Japan Tsunemi Kubodera Department of Zoology, National Museum of Nature and Science, 3̶ 2 3 ̶ 1 Hyakunin-cho, Shinjuku-ku, Tokyo, 169̶ 0073 Japan E-mail: [email protected] Abstract: The deep-sea cephalopod fauna in the waters off the Pacifi c coast of northeastern Japan was inves- tigated, including benthic continental shelf and slope, and offshore mesopelagic habitats. Cephalopods col- lected by research bottom-trawl were used, and previous studies of deep-sea cephalopod fauna in this region were reviewed, allowing confi rmation of 84 species of cephalopods belonging to 22 families. Subtropical, subarctic and transitional species were represented, and by combining the present survey and published infor- mation, each of these three zones is divided into seven ecological components, characterized by specifi c taxa. Key words: deep-sea, cephalopod fauna, western North Pacifi c, bottom-trawl, mid-water trawl, stomach con- tents of higher predators, ecology Introduction The waters off the Pacifi c coast of northeastern Japan are some of the most productive fi shing grounds in the world. The richness of sea-life in this region is supported by large-scale mixing of the cold Oyashio Current from the north and the warm Kuroshio Current from the south; among others, substantial pelagic fi sheries for the Japanese common and neon fl ying squids have devel- oped.