Galvanic Compatibility for Bal™ Seal Spring Materials
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Aero-Flex Corporation 3147 Jupiter Park Circle Suite 2 Jupiter, Florida 33458 (561) 745-2534
Aero-Flex Corporation 3147 Jupiter Park Circle Suite 2 Jupiter, Florida 33458 (561) 745-2534 QUALITY WITHOUT QUESTION Flexible Metal Hose Assemblies Corrugated Metal Hoses that we offer: Hose Master Hoses OmegaFlex Hoses Penflex Hoses ANNUFLEX 700 Series Abrasion Resistant Tubular Braid for 1SBX Braid for 700 Series Hose Annuflex 700 Series Series 300 Hose- Stainless Steel Bronze Braid T304 Bronzeflex 1100 Series Monel Braid Bronze Tubular Braid for Series 400 ChemKing™ Hose Series 400 Stainless Steel Hose Monel Tubular Braid for Series 500 Series 600 Stainless Steel Braid ChlorSafe™ Hose Series 600 Stainless Steel Hose Series 100 - Helical, Stainless Steel, Series 700 Stainless Steel and Extraflex 9000 Series Standard Pitch Hose Compressed Hose Formaflex 900 Series Series 300 - Annular, Stainless Steel, Series 740 Monel Hose Standard Pitch Hose Series 794 Bronze Hose Hydraflex 9400 Series Series 400 - Annular, Bronze, Standard Pitch Hose Series 800 High Pressure Braid Interflex Series 800 Stainless Steel Hose Series 500 - Annular, Monel, Standard Pitch Hose Series 900 High Pressure Braid Masterflex 500 Series Series 900 Stainless Steel Hose Series 800 - Annular, Stainless Steel, Pressureflex HP High Pressure Hose and Braid Series P3 Stainless Steel Braid PressureMax HP Series P3 Stainless Steel Hose Tubular Braid for Series 100 Hose - Stainless Steel T304, T321 and T316 Stainless Steel Braid for Series Tar and Asphalt 400 Helical Hose Tubular Braid for Series 300 Stainless Steel Standard Braid for Ultraflex Braided Braid for Series 300 Series 700 Hose 1/4 - 12 inch 304, 316, & 321 Stainless Steel Single or Double Braid Why Flex Hose? It is comparatively light weight, temperature resistance, has great pressure retention, and allows for variance in fit up and alignment. -

Elemental Fluorine Product Information (Pdf)
Elemental Fluorine Contents 1 Introduction ............................................................................................................... 4 2.1 Technical Application of Fluorine ............................................................................. 5 2.2 Electronic Application of Fluorine ........................................................................... 7 2.3 Fluorine On-Site Plant ............................................................................................ 8 3 Specifications ............................................................................................................ 9 4 Safety ...................................................................................................................... 10 4.1 Maintenance of the F2 system .............................................................................. 12 4.2 First Aid ................................................................................................................ 13 5.1 Chemical Properties ............................................................................................. 14 5.2 Physical Data ....................................................................................................... 15 6 Toxicity .................................................................................................................... 18 7 Shipping and Transport ........................................................................................... 20 8 Environment ........................................................................................................... -

The Periodic Table of Elements
The Periodic Table of Elements 1 2 6 Atomic Number = Number of Protons = Number of Electrons HYDROGENH HELIUMHe 1 Chemical Symbol NON-METALS 4 3 4 C 5 6 7 8 9 10 Li Be CARBON Chemical Name B C N O F Ne LITHIUM BERYLLIUM = Number of Protons + Number of Neutrons* BORON CARBON NITROGEN OXYGEN FLUORINE NEON 7 9 12 Atomic Weight 11 12 14 16 19 20 11 12 13 14 15 16 17 18 SODIUMNa MAGNESIUMMg ALUMINUMAl SILICONSi PHOSPHORUSP SULFURS CHLORINECl ARGONAr 23 24 METALS 27 28 31 32 35 40 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 POTASSIUMK CALCIUMCa SCANDIUMSc TITANIUMTi VANADIUMV CHROMIUMCr MANGANESEMn FeIRON COBALTCo NICKELNi CuCOPPER ZnZINC GALLIUMGa GERMANIUMGe ARSENICAs SELENIUMSe BROMINEBr KRYPTONKr 39 40 45 48 51 52 55 56 59 59 64 65 70 73 75 79 80 84 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 RUBIDIUMRb STRONTIUMSr YTTRIUMY ZIRCONIUMZr NIOBIUMNb MOLYBDENUMMo TECHNETIUMTc RUTHENIUMRu RHODIUMRh PALLADIUMPd AgSILVER CADMIUMCd INDIUMIn SnTIN ANTIMONYSb TELLURIUMTe IODINEI XeXENON 85 88 89 91 93 96 98 101 103 106 108 112 115 119 122 128 127 131 55 56 72 73 74 75 76 77 78 79 80 81 82 83 84 85 86 CESIUMCs BARIUMBa HAFNIUMHf TANTALUMTa TUNGSTENW RHENIUMRe OSMIUMOs IRIDIUMIr PLATINUMPt AuGOLD MERCURYHg THALLIUMTl PbLEAD BISMUTHBi POLONIUMPo ASTATINEAt RnRADON 133 137 178 181 184 186 190 192 195 197 201 204 207 209 209 210 222 87 88 104 105 106 107 108 109 110 111 112 113 114 115 116 117 118 FRANCIUMFr RADIUMRa RUTHERFORDIUMRf DUBNIUMDb SEABORGIUMSg BOHRIUMBh HASSIUMHs MEITNERIUMMt DARMSTADTIUMDs ROENTGENIUMRg COPERNICIUMCn NIHONIUMNh -

Llfltfits Paul Stanek, MST-6
COAJF- 9 50cDo/-~^0 LA-UR- 9 5-1666 Title: AGE HARDENING IN RAPIDLY SOLIDIFIED AND HOT ISOSTATICALLY PRESSED BERYLLIUM-ALUM1UM SILVER ALLOYS HVAUli Author(s): David H. Carter, MST-6 Andrew McGeorge, MST-6 Loren A. Jacobson, MST-6 llfltfits Paul Stanek, MST-6 IflllS,Ic 8-o° ,S8 si Proceedings of TMS Annual Meeting, Las Vegas, jJffilJSif—- Nevada, February 1995 as-IVUiS' i in Los Alamos NATIONAL LABORATORY Los Alamos National Laboratory, an affirmative action/equal opportunity employer, is operated by the University of California for the U.S. Department of Energy under contract W-7405-ENG-36. By acceptance of this article, the publisher recognizes that the U.S. Government retains a nonexclusive, royalty-free license to publish or reproduce the published form of this contribution, or to allow others to do so, for U.S. Government purposes. The Los Alamos National Laboratory requests that the publisher identify this article as work performed under the auspices of the U.S. Department of Energy* FotmNo.B36R5 MCTOIQimnM f\c mie hAMMr&T 10 i mi in ST26291M1 DISCLAIMER Portions of this document may be illegible in electronic image products. Images are produced from the best available original document. Age Hardening in Rapidly Solidified and Hot Isostatically Pressed Beryllium-Aluminum-Silver Alloys David H. Carter, Andrew C. McGeorge,* Loren A. Jacobson, and Paul W. Stanek Los Alamos National Laboratory, Los Alamos NM 87545 Abstract Three different alloys of beryllium, aluminum and silver were processed to powder by centrifugal atomization in a helium atmosphere. Alloy com• positions were, by weight, 50% Be, 47.5% Al, 2.5% Ag, 50% Be, 47% Al, 3% Ag, and 50% Be, 46% Al, 4% Ag. -

Of the Periodic Table
of the Periodic Table teacher notes Give your students a visual introduction to the families of the periodic table! This product includes eight mini- posters, one for each of the element families on the main group of the periodic table: Alkali Metals, Alkaline Earth Metals, Boron/Aluminum Group (Icosagens), Carbon Group (Crystallogens), Nitrogen Group (Pnictogens), Oxygen Group (Chalcogens), Halogens, and Noble Gases. The mini-posters give overview information about the family as well as a visual of where on the periodic table the family is located and a diagram of an atom of that family highlighting the number of valence electrons. Also included is the student packet, which is broken into the eight families and asks for specific information that students will find on the mini-posters. The students are also directed to color each family with a specific color on the blank graphic organizer at the end of their packet and they go to the fantastic interactive table at www.periodictable.com to learn even more about the elements in each family. Furthermore, there is a section for students to conduct their own research on the element of hydrogen, which does not belong to a family. When I use this activity, I print two of each mini-poster in color (pages 8 through 15 of this file), laminate them, and lay them on a big table. I have students work in partners to read about each family, one at a time, and complete that section of the student packet (pages 16 through 21 of this file). When they finish, they bring the mini-poster back to the table for another group to use. -

Stainless Steel Selection Guide
TAContentsBLE OF Discovery of Stainless Steel . .page 2 What is Stainless Steel . .page 3 Stainless Steel Classifications . .page 4 • Austenitic . .page 4 • Ferritic . .page 5 • Duplex . .page 5 • Martensitic . .page 6 • Precipitation Hardening . .page 6 Nickel Based Alloys . .page 7 Strength & Heat Treatment . .page 7 The Basics of Corrosion . .page 8 • General or Uniform Corrosion . .page 9 • Galvanic Corrosion . .page 11 • Pitting Corrosion . .page 11 • Crevice Corrosion . .page 13 • Intergranular Corrosion . .page 14 • Stress Corrosion Cracking . .page 15 • Microbiologically Influence Corrosion . .page 17 Welding Stainless Steel . .page 18 Alloy Selection . .page 22 Wrought Stainless Steel Composition . .page 24 Wrought Nickel Alloy Composition . .page 25 Stainless Steel and Nickel Alloy Filler Metal . .page 26 PR“It’sEF stainlessAC steel,E it shouldn’t rust” This is often the kind of statements heard from individuals when discussing a failure of process piping or equipment. This is also an indication of how little is actually understood about stainless steel and the applications where it is used. For years the food, beverage and pharmaceutical industries have used stainless steels in their process piping systems. Most of the time stainless steel components provide satisfactory results. Occasionally a catastrophic failure will occur. The purpose of the information contained within this document is to bring an understanding to stainless steel, it’s uses, and why it will fail under certain conditions. In the following pages we will discuss the different classes of stainless steel, heat treatment, corrosion, welding, and finally material selection. As with any failure, it is imperative the cause of the failure be identified before a proper fix can be recognized. -
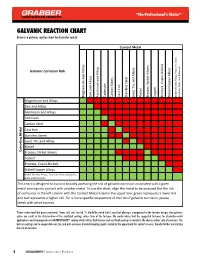
GALVANIC REACTION CHART Below Is a Galvanic Reaction Chart for Dissimilar Metals
GALVANIC REACTION CHART Below is a galvanic reaction chart for dissimilar metals. Contact Metal Silver, Silver, Galvanic Corrosion Risk Alloys Alloys Platinum Alloys Alloys, Titanium, and Alloys Steels Gold, and Iron Cast Stainless Steel Lead, Tin, and Magnesium and Zinc and Cadmium Carbon Nickel Brasses, Nickel-Silvers Copper Bronzes, Cupro-Nickels Nickel Copper Alloys Aluminum Graphite, Nickel-Chrome Magnesium and Alloys Zinc and Alloys Aluminum and Alloys Cadmium Carbon Steel Cast Iron Metal Stainless Steels Lead, Tin, and Alloys Nickel Corroding Brasses, Nickel-Silvers Copper Bronzes, Cupro-Nickels Nickel Copper Alloys Nickel-Chrome Alloys, Titanium, Silver, Graphite, Gold, and Platinum This chart is designed to assist in broadly assessing the risk of galvanic corrosion associated with a given metal coming into contact with another metal. To use the chart, align the metal to be assessed (for the risk of corrosion) in the left column with the Contact Metal listed in the upper row; green represents a lower risk and red represents a higher risk. For a more specific assessment of the risk of galvanic corrosion, please check with other sources. Please understand that green represents "lower risk" not "no risk." It should be noted that if sacrificial plating is incorporated in the fastener design, then galvanic action can result in the deterioration of the sacrificial coating, rather than of the fastener. We would advise that the suggested fasteners for dissimilar-metal applications would incorporate our GRABBERGARD® coating which utilizes both barrier and sacrificial coatings to minimize the chance and/or rate of corrosion. The barrier coating used to encapsulate our zinc and anti-corrosion chemical bonding agents minimize the opportunity for contact to occur, thereby further minimizing the risk of corrosion. -

Galvanic Corrosion
10 GALVANIC CORROSION X. G. ZHANG Teck Metals Ltd., Mississauga, Ontario, Canada A. Introduction graphite, are dispersed in a metal, or on a ship, where the B. Definition various components immersed in water are made of different C. Factors in galvanic corrosion metal alloys. In many cases, galvanic corrosion may result in D. Material factors quick deterioration of the metals but, in other cases, the D1. Effects of coupled materials galvanic corrosion of one metal may result in the corrosion D2. Effect of area protection of an attached metal, which is the basis of cathodic D3. Effect of surface condition protection by sacrificial anodes. E. Environmental factors Galvanic corrosion is an extensively investigated subject, E1. Effects of solution as shown in Table 10.1, and is qualitatively well understood E2. Atmospheric environments but, due to its highly complex nature, it has been difficult to E3. Natural waters deal with in a quantitative way until recently. The widespread F. Polarity reversal use of computers and the development of software have made G. Preventive measures great advances in understanding and predicting galvanic H. Beneficial effects of galvanic corrosion corrosion. I. Fundamental considerations I1. Electrode potential and Kirchhoff’s law I2. Analysis B. DEFINITION I3. Polarization and resistance I4. Potential and current distributions When two dissimilar conducting materials in electrical con- References tact with each other are exposed to an electrolyte, a current, called the galvanic current, flows from one to the other. Galvanic corrosion is that part of the corrosion that occurs at the anodic member of such a couple and is directly related to the galvanic current by Faraday’s law. -

Beryllium Compounds (A) BERYLLIUM COMPOUNDS
Beryllium Compounds (A) BERYLLIUM COMPOUNDS 107-02-8 Hazard Summary Inhalation exposure to beryllium primarily occurs in the workplaces where it is mined, processed, or converted into alloys and chemicals, or from the burning of coal or fuel oil and in tobacco smoke. Acute (short-term) inhalation exposure to high levels of beryllium has been observed to cause inflammation of the lungs or acute pneumonitis (reddening and swelling of the lungs) in humans; after exposure ends, these symptoms may be reversible. Chronic (long-term) inhalation exposure of humans to beryllium has been reported to cause chronic beryllium disease (berylliosis), in which granulomatous lesions (noncancerous) develop in the lung. Human epidemiology studies are limited, but suggest a causal relationship between beryllium exposure and an increased risk of lung cancer. Inhalation exposure to beryllium has been demonstrated to cause lung cancer in rats and monkeys. EPA has classified beryllium as a Group B1, probable human carcinogen. Please Note: The main sources of information for this fact sheet are EPA's Integrated Risk Information System (IRIS) (3), which contains information on oral chronic toxicity and the RfD and inhalation chronic toxicity and the RfC, and the carcinogenic effects of beryllium including the unit cancer risk for inhalation exposure, EPA's Toxicological Review of Beryllium and Compounds (2), and the Agency for Toxic Substances and Disease Registry's (ATSDR's) Toxicological Profile for Beryllium. (1) Uses Pure beryllium and its metal alloys have applications in electrical components, tools, structural components for aircraft, missiles, and satellites, and other metal-fabricating uses. (1) Beryllium is also used in consumer products, such as televisions, calculators, and personal computers. -

Galvanic Corrosion Final
GALVANIC CORROSION by Stephen C. Dexter, Professor of Applied Science and Marine Biology, (302) 645-4261 Galvanic corrosion, often misnamed “electrolysis,” is one common form of corrosion in marine environments. It occurs Table 1 when two (or more) dissimilar metals are brought into electri- GALVANIC SERIES cal contact under water. When a galvanic couple forms, one of In Flowing Seawater the metals in the couple becomes the anode and corrodes faster than it would all by itself, while the other becomes the cathode Voltage Range of Alloy and corrodes slower than it would alone. Either (or both) metal Alloy vs. Reference Electrode* in the couple may or may not corrode by itself (themselves) in MagnesiumAnodic or -1.60 to -1.63 seawater. When contact with a dissimilar metal is made, how- Active End ever, the self-corrosion rates will change: corrosion of the Zinc -0.98 to -1.03 anode will accelerate; corrosion of the cathode will decelerate Aluminum Alloys -0.70 to -0.90 or even stop. We can use the seawater Galvanic Series, shown Cadmium -0.70 to -0.76 in Table 1, to predict which metal will become the anode and Cast Irons -0.60 to -0.72 how rapidly it will corrode. Steel -0.60 to -0.70 Aluminum Bronze -0.30 to -0.40 The seawater Galvanic Series is a list of metals and alloys Red Brass, Yellow Brass, ranked in order of their tendency to corrode in marine environ- Naval Brass -0.30 to -0.40 ments. If any two metals from the list are coupled together, the Copper -0.28 to -0.36 one closer to the anodic (or active) end of the series, the Lead-Tin Solder (50/50) -0.26 to -0.35 upper end in this case, will be the anode and thus will corrode Admiralty Brass -0.25 to -0.34 faster, while the one toward the cathodic (or noble) end will Manganese Bronze -0.25 to -0.33 corrode slower. -

Research a He Edge of Sp Ce Contents I Mission and Accomplishmen Ts
https://ntrs.nasa.gov/search.jsp?R=19640004493 2020-03-11T17:07:03+00:00Z RESEARCH A HE EDGE OF SP CE CONTENTS I MISSION AND ACCOMPLISHMEN TS . .. 1 II EARLIER RESEARCH AIRCRAFT ................. 5 III CONCEPT, HISTORY, AND TECH NICAL CONSIDERATIONS ... 11 IV THE X-15 FLIGH T PROGRAM ................. _ 19 V FUTURE ......... ................... _ 31 ~O';).,IOOO Q. ~ !y f) S 1+) tJ~'(j 1;"v -' ~,I!.-. RESEARCH AT THE EDGE OF SPACE I (/1 5 E F_ '!) CPO ' ~ () I 3 D -1: 1 For sale by the Superintendpnt of Documents, U.S. Government Printing Office 1. ~ ~ : ashington, D.C ., 20402- PriceToCeiits -.-, NASA Ep·9 - - ------- -.--------~~- ~--- .- - -- MISSION AND ACCOMPLISHMENTS , l_ - .-- -- ------ ---- - - I In the fall of 1963, the X-IS completed a series of dental to the goals of the program. The real mis flights at speeds and altitudes never before attained sion of the X-IS is a quest for knowledge. In carry by any vehicle fully controlled by a pilot from launch ing out this mission, the X-IS has made a number to landing on the ground. In the process of making of substantial contributions to the advancement of almost 100 successful flights, it had essentially aerospace science. completed its original program of fli ght research Although primarily an aeronautical vehicle with and had begun to carry out additional aerospace wings and aerodynamic controls, the aircraft travels experiments. well beyond the effective atmosphere on most of its From conception through the phases of design, flights_ At extreme altitudes, the pilot controls the construction, test, and operation, it had rounded out X-IS by reaction jets, like a spacecraft; he is some 11 years of exploring a variety of technological weightless for brief periods, and the research plane and scientific problems. -

Galvanic Corrosion Between Zinc and Carbon Steel Investigated by Local
Galvanic corrosion between zinc and carbon steel investigated by local electrochemical impedance spectroscopy Maixent Mouanga, Monique Puiggali, Bernard Tribollet, Vincent Vivier, Nadine Pébère, Olivier Devos To cite this version: Maixent Mouanga, Monique Puiggali, Bernard Tribollet, Vincent Vivier, Nadine Pébère, et al.. Gal- vanic corrosion between zinc and carbon steel investigated by local electrochemical impedance spec- troscopy. Electrochimica Acta, Elsevier, 2013, 88, pp.6-14. 10.1016/j.electacta.2012.10.002. hal- 01165531 HAL Id: hal-01165531 https://hal.archives-ouvertes.fr/hal-01165531 Submitted on 19 Jun 2015 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Open Archive TOULOUSE Archive Ouverte ( OATAO ) OATAO is an open access repository that collects the work of Toulouse researchers and makes it freely available over the web where possible. This is an author-deposited version published in : http://oatao.univ-toulouse.fr/ Eprints ID : 14080 To link to this article : doi: 10.1016/j.electacta.2012.10.002 URL : http://dx.doi.org/10.1016/j.electacta.2012.10.002 To cite this version : Mouanga, Maixent and Puiggali, Monique and Tribollet, Bernard and Vivier, Vincent and Pébère, Nadine and Devos, Olivier Galvanic corrosion between zinc and carbon steel investigated by local electrochemical impedance spectroscopy .