Galvanic Corrosion
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cathodic Protection of Concrete Bridges: a Manual of Practice
SHRP-S-372 Cathodic Protection of Concrete Bridges: A Manual of Practice J. E. Bennett John J. Bartholomew ELTECH Research Corporation Fairport Harbor, Ohio James B. Bushman Bushman & Associates Medina, Ohio Kenneth C. Clear K. C. Clear, Inc. Boston, Virginia Robert N. Kamp Consulting Engineer Albany, New York Wayne J. Swiat Corrpro Companies, Inc. Medina, Ohio Strategic Highway Research Program National Research Council Washington, DC 1993 SHRP-S-372 ISBN 0-309-05750-7 Contract C-102D Product No. 2034 Program Manager: Don M. Harriott Project Manager: It. Martin (Marty) Laylor Consultant: John P. Broomfield Production Editor: Cara J. Tate Program Area Secretary: Carina S. Hreib December 1993 key words: bridges bridge maintenance bridge rehabilitation cathodic protection chlorides corrosion corrosion prevention corrosion rate CP electrochemical methods reinforced concrete Strategic Highway Research Program National Academy of Sciences 2101 Constitution Avenue N.W. Washington, DC 20418 (202) 334-3774 The publication of this report does not necessarily indicate approval or endorsement of the findings, opinions, conclusions, or recommendations either inferred or specifically expressed herein by the National Academy of Sciences, the United States Government, or the American Association of State Highway and Transportation Officials or its member states. © 1993 National A_zademy of Sciences I.SM/NAP/1293 Acknowledgments The research described herein was supported by the Strategic Highway Research Program (SHRP). SHRP is a unit of the National Research Council that was authorized by section 128 of the Surface Transportation and Uniform Relocation Assistance Act of 1987. This report is a compilation of work by ELTECH Research Corporation, Corrpro Companies, Inc., and Kenneth C. -

GALVANIC/DISSIMILAR METAL CORROSION What It Is and How to Avoid It ASSDA Technical FAQ No 1 1 Edition 2, May 2009
AUSTRALIAN STAINLESS STEEL DEVELOPMENT ASSOCIATION GALVANIC/DISSIMILAR METAL CORROSION What it is and how to avoid it ASSDA Technical FAQ No 1 1 Edition 2, May 2009 Contact between dissimilar metals Metal to metal contact The graph shows that stainless steels have occurs frequently but is often not two ranges of potential. The usual, passive Galvanic corrosion can only occur if the a problem. The aluminium head behaviour is shown by the light hatching. dissimilar metals are in electrical contact. However, if the passive film breaks down, on a cast iron block, the solder on The contact may be direct or by an the stainless steel corrodes and its a copper pipe, galvanising on a external pipe or wire or bolt. If the potential is in the dark bar range. steel purlin and the steel fastener dissimilar metals are insulated from each in an aluminium sheet are common other by suitable plastic strips, washers or As a rule of thumb, if the potential examples. sleeves then galvanic corrosion cannot difference is less than 0.1 volt, then it is occur. Paint is not a reliable electrical unlikely that galvanic corrosion will be significant. WHAT CAUSES GALVANIC insulator especially under bolt heads or CORROSION? nuts or washers or near edges of sheets of If all three conditions are met then metal. The paint is usually damaged on galvanic corrosion is probable and the rate For galvanic or dissimilar or installation or by subsequent movement. of corrosion will be influenced by the electrolytic corrosion to occur, Note that the chromium oxide film layer relative area and the current density three conditions must be met: on the stainless steel is very thin and not delivered by the noble metal. -

Humectants to Augment Current from Metallized Zinc Cathodic Protection Systems on Concrete
HUMECTANTS TO AUGMENT CURRENT FROM METALLIZED ZINC CATHODIC PROTECTION SYSTEMS ON CONCRETE Final Report SPR 384 HUMECTANTS TO AUGMENT CURRENT FROM METALLIZED ZINC CATHODIC PROTECTION SYSTEMS ON CONCRETE Final Report SPR 384 by Gordon R. Holcomb, Bernard S. Covino, Jr., Stephen D. Cramer, James H. Russell, Sophie J. Bullard, and W. Keith Collins Albany Research Center, U. S. Department of Energy, Albany OR 97321 Jack E. Bennett J. E. Bennett Consulting, Inc., Chardon OH 44024 Steven M. Soltesz and H. Martin Laylor Oregon Department of Transportation, Salem OR 97301 for Oregon Department of Transportation, Research Group 200 Hawthorne SE, Suite B-240 Salem OR 97301-5192 and Federal Highway Administration Washington, D.C. December 2002 Technical Report Documentation Page 1. Report No. 2. Government Accession No. 3. Recipient’s Catalog No. FHWA-OR-RD-03-08 4. Title and Subtitle 5. Report Date Humectants to Augment Current from Metallized Zinc Cathodic Protection December 2002 Systems on Concrete 6. Performing Organization Code 7. Author(s) 8. Performing Organization Report No. Gordon R. Holcomb, Bernard S. Covino, Jr., Stephen D. Cramer, James H. Russell, Sophie J. Bullard, and W. Keith Collins Albany Research Center, U. S. Department of Energy, Albany OR 97321 Jack E. Bennett, J. E. Bennett Consulting, Inc., Chardon OH 44024 Steven M. Soltesz and H. Martin Laylor, Oregon Department of Transportation, Salem OR 97301 9. Performing Organization Name and Address 10. Work Unit No. (TRAIS) Oregon Department of Transportation Research Group 11. Contract or Grant No. 200 Hawthorne Avenue SE, Suite B-240 SPR 384 Salem, Oregon 97301-5192 12. -

Corrosion Control Plan for Bridges
Contents Introduction There is essentially no argument that the American infrastructure is in Introduction ................................................. i poor shape and there is little indication that significant improvement is on the horizon. Acknowledgments ....................................ii The amount of money needed to correct this problem is Executive Summary ..................................2 staggering, especially considering the current state of the economy. Crumbling Infrastructure ........................4 One reason for this is the age profile of the nation’s bridges. Figure 1 1 Introduction to Corrosion ......................6 shows this profile taken from the 2010 National Bridge Inventory . It shows bridges are approaching the maximum age distribution of Bridges to Everywhere ............................7 around 50 years. Most bridges were built for a 50 year design life, which means state highway departments will have to maintain those Corrosion Basics ...................................... 11 bridges beyond their original design lives, which will be challenging because they were built to lower design standards than those used Corrosion in Concrete ........................... 14 today. Exposure Conditions ............................. 18 Corrosion Control ................................... 21 The Highway Ahead .............................. 27 References ................................................. 29 Figure 1: Distribution of bridges by age (2010 NBI data) When the Eisenhower Interstate System was created, -

Analysis of Alternatives
ANALYSIS OF ALTERNATIVES Legal name of applicants: Henkel AG & Co. KGaA; Henkel Global Supply Chain B.V. Submitted by: Henkel AG & Co. KGaA. Substance: Dichromium tris(chromate), EC No: 246-356-2, CAS No: 24613-89-6 Use title: Use of Dichromium tris(chromate) for surface treatment of metals such as aluminium, steel, zinc, magnesium, titanium, alloys, composites, sealings of anodic films Use number: 2 Use number: 2 Copy right protected - Property of Members of the CCST Consortium - No copying / use allowed. ANALYSIS OF ALTERNATIVES Disclaimer This document shall not be construed as expressly or implicitly granting a license or any rights to use related to any content or information contained therein. In no event shall applicant be liable in this respect for any damage arising out or in connection with access, use of any content or information contained therein despite the lack of approval to do so. ii Use number: 2 Copy right protected - Property of Members of the CCST Consortium - No copying / use allowed. ANALYSIS OF ALTERNATIVES CONTENTS DECLARATION .......................................................................................................................................................... XV 1. SUMMARY 1 2. INTRODUCTION .................................................................................................................................................. 8 2.1. Substances ........................................................................................................................................ 8 2.2. -
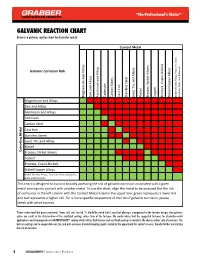
GALVANIC REACTION CHART Below Is a Galvanic Reaction Chart for Dissimilar Metals
GALVANIC REACTION CHART Below is a galvanic reaction chart for dissimilar metals. Contact Metal Silver, Silver, Galvanic Corrosion Risk Alloys Alloys Platinum Alloys Alloys, Titanium, and Alloys Steels Gold, and Iron Cast Stainless Steel Lead, Tin, and Magnesium and Zinc and Cadmium Carbon Nickel Brasses, Nickel-Silvers Copper Bronzes, Cupro-Nickels Nickel Copper Alloys Aluminum Graphite, Nickel-Chrome Magnesium and Alloys Zinc and Alloys Aluminum and Alloys Cadmium Carbon Steel Cast Iron Metal Stainless Steels Lead, Tin, and Alloys Nickel Corroding Brasses, Nickel-Silvers Copper Bronzes, Cupro-Nickels Nickel Copper Alloys Nickel-Chrome Alloys, Titanium, Silver, Graphite, Gold, and Platinum This chart is designed to assist in broadly assessing the risk of galvanic corrosion associated with a given metal coming into contact with another metal. To use the chart, align the metal to be assessed (for the risk of corrosion) in the left column with the Contact Metal listed in the upper row; green represents a lower risk and red represents a higher risk. For a more specific assessment of the risk of galvanic corrosion, please check with other sources. Please understand that green represents "lower risk" not "no risk." It should be noted that if sacrificial plating is incorporated in the fastener design, then galvanic action can result in the deterioration of the sacrificial coating, rather than of the fastener. We would advise that the suggested fasteners for dissimilar-metal applications would incorporate our GRABBERGARD® coating which utilizes both barrier and sacrificial coatings to minimize the chance and/or rate of corrosion. The barrier coating used to encapsulate our zinc and anti-corrosion chemical bonding agents minimize the opportunity for contact to occur, thereby further minimizing the risk of corrosion. -

Development of a Low Resistance Low Corrosion Cathode Plate
Development of a low resistance, low corrosion cathode plate for electrowinning and electrorefining Nigel J. Aslin1*1, Christian Pasten2, Addin Pranowo3, Graham J. Heferen4 1. Glencore Technology, Australia ABSTRACT For nearly 40 years Glencore Technology (formerly Mount Isa Mines Copper Refineries) has been the mainstay supplier of permanent cathode plates to the copper industry with its ISA PROCESS and KIDD PROCESS cathode plates. Improvement to the cathode plate design remains a key area for research, and on-going developments by Glencore Technology have led to the commercialisation of a new design. The ISAKIDD cathode plate is universally suited to both electro-refining and electro- winning bringing major cost efficiencies to the operations through improvements in hanger bar strength, corrosion resistance and electrical conductivity. A variation of the this design uses a ‘steer- horn’ shaped hanger bar to lower the resistance path across the cathode plate giving significant tank- house power savings in electrowinning refineries. Technical aspects and results of commercial trials of the ISAKIDD and ‘HP’ cathode plate are discussed in this paper. A novel new contact system which allows shorting frames to be used with Stainless Steel hanger bars will also be tested. Trial results will demonstrate savings in a typical EW tankhouse of up to 720k USD per year in power costs alone, with further savings in maintenance costs and operational efficiencies. *Corresponding author: Glencore Technology, Technical Manager, c/- Copper Refineries Pty Ltd, Hunter St, Stuart, 4811, QLD, Australia. Phone: +61 418887034. Email: [email protected] 1 INTRODUCTION Glencore Technology were the pioneers of Permanent cathode technology for copper and remain committed to continuous improvement and innovation of the technology forty years later. -

Galvanic Corrosion Final
GALVANIC CORROSION by Stephen C. Dexter, Professor of Applied Science and Marine Biology, (302) 645-4261 Galvanic corrosion, often misnamed “electrolysis,” is one common form of corrosion in marine environments. It occurs Table 1 when two (or more) dissimilar metals are brought into electri- GALVANIC SERIES cal contact under water. When a galvanic couple forms, one of In Flowing Seawater the metals in the couple becomes the anode and corrodes faster than it would all by itself, while the other becomes the cathode Voltage Range of Alloy and corrodes slower than it would alone. Either (or both) metal Alloy vs. Reference Electrode* in the couple may or may not corrode by itself (themselves) in MagnesiumAnodic or -1.60 to -1.63 seawater. When contact with a dissimilar metal is made, how- Active End ever, the self-corrosion rates will change: corrosion of the Zinc -0.98 to -1.03 anode will accelerate; corrosion of the cathode will decelerate Aluminum Alloys -0.70 to -0.90 or even stop. We can use the seawater Galvanic Series, shown Cadmium -0.70 to -0.76 in Table 1, to predict which metal will become the anode and Cast Irons -0.60 to -0.72 how rapidly it will corrode. Steel -0.60 to -0.70 Aluminum Bronze -0.30 to -0.40 The seawater Galvanic Series is a list of metals and alloys Red Brass, Yellow Brass, ranked in order of their tendency to corrode in marine environ- Naval Brass -0.30 to -0.40 ments. If any two metals from the list are coupled together, the Copper -0.28 to -0.36 one closer to the anodic (or active) end of the series, the Lead-Tin Solder (50/50) -0.26 to -0.35 upper end in this case, will be the anode and thus will corrode Admiralty Brass -0.25 to -0.34 faster, while the one toward the cathodic (or noble) end will Manganese Bronze -0.25 to -0.33 corrode slower. -

Brad A. James, Ph.D., P.E., FASM
Brad A. James, Ph.D., P.E., FASM Group Vice President & Principal Engineer | Materials & Corrosion Engineering 149 Commonwealth Drive | Menlo Park, CA 94025 (650) 688-7391 tel | [email protected] Professional Profile Dr. James specializes in failure analysis, failure prevention, and integrity assessment of engineering structures and components. His specific expertise includes metallurgy, materials science, fracture, fatigue, material degradation, corrosion, life prediction, and design. In his many years of engineering experience, Dr. James has conducted thousands of failure analysis investigations on widely varying engineering structures, ranging from miniscule medical devices to power- plant components. Dr. James also helps clients from various industries prevent failures, assess the integrity of their designs or equipment, as well as interact with governmental agencies. Dr. James has special interest in fractography, fracture mechanics, wear, corrosion, embrittlement phenomena, microstructural development, heat treatment, material selection, and welding and joining. The common thread in each of Dr. James' investigations is the application of metallurgical, materials science, and engineering mechanics fundamentals to help understand and solve complex problems. Dr. James has taught several graduate-level fracture mechanics and failure analysis courses at Stanford and Santa Clara Universities. He has also taught several courses for The American Society for Materials (ASM International) involving failure analysis, design, and life prediction/validation -

Galvanic Corrosion Between Zinc and Carbon Steel Investigated by Local
Galvanic corrosion between zinc and carbon steel investigated by local electrochemical impedance spectroscopy Maixent Mouanga, Monique Puiggali, Bernard Tribollet, Vincent Vivier, Nadine Pébère, Olivier Devos To cite this version: Maixent Mouanga, Monique Puiggali, Bernard Tribollet, Vincent Vivier, Nadine Pébère, et al.. Gal- vanic corrosion between zinc and carbon steel investigated by local electrochemical impedance spec- troscopy. Electrochimica Acta, Elsevier, 2013, 88, pp.6-14. 10.1016/j.electacta.2012.10.002. hal- 01165531 HAL Id: hal-01165531 https://hal.archives-ouvertes.fr/hal-01165531 Submitted on 19 Jun 2015 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Open Archive TOULOUSE Archive Ouverte ( OATAO ) OATAO is an open access repository that collects the work of Toulouse researchers and makes it freely available over the web where possible. This is an author-deposited version published in : http://oatao.univ-toulouse.fr/ Eprints ID : 14080 To link to this article : doi: 10.1016/j.electacta.2012.10.002 URL : http://dx.doi.org/10.1016/j.electacta.2012.10.002 To cite this version : Mouanga, Maixent and Puiggali, Monique and Tribollet, Bernard and Vivier, Vincent and Pébère, Nadine and Devos, Olivier Galvanic corrosion between zinc and carbon steel investigated by local electrochemical impedance spectroscopy . -

The Effects and Economic Impact of Corrosion
© 2000 ASM International. All Rights Reserved. www.asminternational.org Corrosion: Understanding the Basics (#06691G) CHAPTER 1 The Effects and Economic Impact of Corrosion CORROSION is a natural process. Just like water flows to the lowest level, all natural processes tend toward the lowest possible energy states. Thus, for example, iron and steel have a natural tendency to com- bine with other chemical elements to return to their lowest energy states. In order to return to lower energy states, iron and steel frequently combine with oxygen and water, both of which are present in most natu- ral environments, to form hydrated iron oxides (rust), similar in chemi- cal composition to the original iron ore. Figure 1 illustrates the corro- sion life cycle of a steel product. Finished Steel Product Air & Moisture Corrode Steel & Form Smelting & Rust Refining Adding Giving Up Energy Energy Mining Ore Iron Oxide (Ore & Rust) Fig. 1 The corrosion cycle of steel © 2000 ASM International. All Rights Reserved. www.asminternational.org Corrosion: Understanding the Basics (#06691G) 2 Corrosion: Understanding the Basics The Definition of Corrosion Corrosion can be defined in many ways. Some definitions are very narrow and deal with a specific form of corrosion, while others are quite broad and cover many forms of deterioration. The word corrode is de- rived from the Latin corrodere, which means “to gnaw to pieces.” The general definition of corrode is to eat into or wear away gradually, as if by gnawing. For purposes here, corrosion can be defined as a chemical or electrochemical reaction between a material, usually a metal, and its environment that produces a deterioration of the material and its proper- ties. -

Introduction Engineering 360.826.4570 Nwcorrosion.Com Jeremy Hailey, P.E
Jeremy Hailey Northwest Corrosion Introduction Engineering 360.826.4570 nwcorrosion.com Jeremy Hailey, P.E. • Corrosion Engineer with 16 years experience • Started NW Corrosion Engineering 10 years ago • Focus on corrosion control system designs, troubleshooting, and some installation work • Conduct training seminars and classes for Steel Tank Institute, National Association of Corrosion Engineers, and the American Water Works Ass’n. • NACE Certified Corrosion Specialist, Cathodic Protection Specialist, and Certified Coating Inspector Jeremy Hailey Northwest Corrosion Topics of Discussion Engineering 360.826.4570 nwcorrosion.com 1. Corrosion Theory 2. Methods of Corrosion Control 3. Corrosion Protection Criteria 4. Considerations for Design of Corrosion Control Systems Jeremy Hailey Northwest Corrosion Corrosion Theory Engineering 360.826.4570 nwcorrosion.com All materials have various physical properties – Color, hardness, ductility, shear strength, ability to conduct heat, melting point, electrical potential….. Corrosion in metal occurs because of an electrical imbalance, or electrical potential difference. Much like when two beakers of water, each at a different temperature, are poured together the resulting temperature will be somewhere between the two starting temperatures. Jeremy Hailey Northwest Corrosion Requirements of a Corrosion Cell Engineering 360.826.4570 nwcorrosion.com For corrosion to occur, four individual items must be present: 1. Anode – The place where corrosion occurs; the more electronegative site 2. Cathode