Ftsk-Dependent Xercd-Dif Recombination Unlinks Replication Catenanes in a Stepwise Manner
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Ribbon Concordances and Doubly Slice Knots
Ribbon concordances and doubly slice knots Ruppik, Benjamin Matthias Advisors: Arunima Ray & Peter Teichner The knot concordance group Glossary Definition 1. A knot K ⊂ S3 is called (topologically/smoothly) slice if it arises as the equatorial slice of a (locally flat/smooth) embedding of a 2-sphere in S4. – Abelian invariants: Algebraic invari- Proposition. Under the equivalence relation “K ∼ J iff K#(−J) is slice”, the commutative monoid ants extracted from the Alexander module Z 3 −1 A (K) = H1( \ K; [t, t ]) (i.e. from the n isotopy classes of o connected sum S Z K := 1 3 , # := oriented knots S ,→S of knots homology of the universal abelian cover of the becomes a group C := K/ ∼, the knot concordance group. knot complement). The neutral element is given by the (equivalence class) of the unknot, and the inverse of [K] is [rK], i.e. the – Blanchfield linking form: Sesquilin- mirror image of K with opposite orientation. ear form on the Alexander module ( ) sm top Bl: AZ(K) × AZ(K) → Q Z , the definition Remark. We should be careful and distinguish the smooth C and topologically locally flat C version, Z[Z] because there is a huge difference between those: Ker(Csm → Ctop) is infinitely generated! uses that AZ(K) is a Z[Z]-torsion module. – Levine-Tristram-signature: For ω ∈ S1, Some classical structure results: take the signature of the Hermitian matrix sm top ∼ ∞ ∞ ∞ T – There are surjective homomorphisms C → C → AC, where AC = Z ⊕ Z2 ⊕ Z4 is Levine’s algebraic (1 − ω)V + (1 − ω)V , where V is a Seifert concordance group. -

A Knot-Vice's Guide to Untangling Knot Theory, Undergraduate
A Knot-vice’s Guide to Untangling Knot Theory Rebecca Hardenbrook Department of Mathematics University of Utah Rebecca Hardenbrook A Knot-vice’s Guide to Untangling Knot Theory 1 / 26 What is Not a Knot? Rebecca Hardenbrook A Knot-vice’s Guide to Untangling Knot Theory 2 / 26 What is a Knot? 2 A knot is an embedding of the circle in the Euclidean plane (R ). 3 Also defined as a closed, non-self-intersecting curve in R . 2 Represented by knot projections in R . Rebecca Hardenbrook A Knot-vice’s Guide to Untangling Knot Theory 3 / 26 Why Knots? Late nineteenth century chemists and physicists believed that a substance known as aether existed throughout all of space. Could knots represent the elements? Rebecca Hardenbrook A Knot-vice’s Guide to Untangling Knot Theory 4 / 26 Why Knots? Rebecca Hardenbrook A Knot-vice’s Guide to Untangling Knot Theory 5 / 26 Why Knots? Unfortunately, no. Nevertheless, mathematicians continued to study knots! Rebecca Hardenbrook A Knot-vice’s Guide to Untangling Knot Theory 6 / 26 Current Applications Natural knotting in DNA molecules (1980s). Credit: K. Kimura et al. (1999) Rebecca Hardenbrook A Knot-vice’s Guide to Untangling Knot Theory 7 / 26 Current Applications Chemical synthesis of knotted molecules – Dietrich-Buchecker and Sauvage (1988). Credit: J. Guo et al. (2010) Rebecca Hardenbrook A Knot-vice’s Guide to Untangling Knot Theory 8 / 26 Current Applications Use of lattice models, e.g. the Ising model (1925), and planar projection of knots to find a knot invariant via statistical mechanics. Credit: D. Chicherin, V.P. -

On the Number of Unknot Diagrams Carolina Medina, Jorge Luis Ramírez Alfonsín, Gelasio Salazar
On the number of unknot diagrams Carolina Medina, Jorge Luis Ramírez Alfonsín, Gelasio Salazar To cite this version: Carolina Medina, Jorge Luis Ramírez Alfonsín, Gelasio Salazar. On the number of unknot diagrams. SIAM Journal on Discrete Mathematics, Society for Industrial and Applied Mathematics, 2019, 33 (1), pp.306-326. 10.1137/17M115462X. hal-02049077 HAL Id: hal-02049077 https://hal.archives-ouvertes.fr/hal-02049077 Submitted on 26 Feb 2019 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. On the number of unknot diagrams Carolina Medina1, Jorge L. Ramírez-Alfonsín2,3, and Gelasio Salazar1,3 1Instituto de Física, UASLP. San Luis Potosí, Mexico, 78000. 2Institut Montpelliérain Alexander Grothendieck, Université de Montpellier. Place Eugèene Bataillon, 34095 Montpellier, France. 3Unité Mixte Internationale CNRS-CONACYT-UNAM “Laboratoire Solomon Lefschetz”. Cuernavaca, Mexico. October 17, 2017 Abstract Let D be a knot diagram, and let D denote the set of diagrams that can be obtained from D by crossing exchanges. If D has n crossings, then D consists of 2n diagrams. A folklore argument shows that at least one of these 2n diagrams is unknot, from which it follows that every diagram has finite unknotting number. -
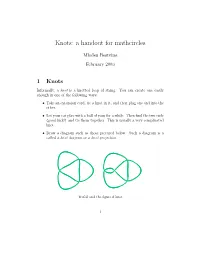
Knots: a Handout for Mathcircles
Knots: a handout for mathcircles Mladen Bestvina February 2003 1 Knots Informally, a knot is a knotted loop of string. You can create one easily enough in one of the following ways: • Take an extension cord, tie a knot in it, and then plug one end into the other. • Let your cat play with a ball of yarn for a while. Then find the two ends (good luck!) and tie them together. This is usually a very complicated knot. • Draw a diagram such as those pictured below. Such a diagram is a called a knot diagram or a knot projection. Trefoil and the figure 8 knot 1 The above two knots are the world's simplest knots. At the end of the handout you can see many more pictures of knots (from Robert Scharein's web site). The same picture contains many links as well. A link consists of several loops of string. Some links are so famous that they have names. For 2 2 3 example, 21 is the Hopf link, 51 is the Whitehead link, and 62 are the Bor- romean rings. They have the feature that individual strings (or components in mathematical parlance) are untangled (or unknotted) but you can't pull the strings apart without cutting. A bit of terminology: A crossing is a place where the knot crosses itself. The first number in knot's \name" is the number of crossings. Can you figure out the meaning of the other number(s)? 2 Reidemeister moves There are many knot diagrams representing the same knot. For example, both diagrams below represent the unknot. -

Knot Theory and the Alexander Polynomial
Knot Theory and the Alexander Polynomial Reagin Taylor McNeill Submitted to the Department of Mathematics of Smith College in partial fulfillment of the requirements for the degree of Bachelor of Arts with Honors Elizabeth Denne, Faculty Advisor April 15, 2008 i Acknowledgments First and foremost I would like to thank Elizabeth Denne for her guidance through this project. Her endless help and high expectations brought this project to where it stands. I would Like to thank David Cohen for his support thoughout this project and through- out my mathematical career. His humor, skepticism and advice is surely worth the $.25 fee. I would also like to thank my professors, peers, housemates, and friends, particularly Kelsey Hattam and Katy Gerecht, for supporting me throughout the year, and especially for tolerating my temporary insanity during the final weeks of writing. Contents 1 Introduction 1 2 Defining Knots and Links 3 2.1 KnotDiagramsandKnotEquivalence . ... 3 2.2 Links, Orientation, and Connected Sum . ..... 8 3 Seifert Surfaces and Knot Genus 12 3.1 SeifertSurfaces ................................. 12 3.2 Surgery ...................................... 14 3.3 Knot Genus and Factorization . 16 3.4 Linkingnumber.................................. 17 3.5 Homology ..................................... 19 3.6 TheSeifertMatrix ................................ 21 3.7 TheAlexanderPolynomial. 27 4 Resolving Trees 31 4.1 Resolving Trees and the Conway Polynomial . ..... 31 4.2 TheAlexanderPolynomial. 34 5 Algebraic and Topological Tools 36 5.1 FreeGroupsandQuotients . 36 5.2 TheFundamentalGroup. .. .. .. .. .. .. .. .. 40 ii iii 6 Knot Groups 49 6.1 TwoPresentations ................................ 49 6.2 The Fundamental Group of the Knot Complement . 54 7 The Fox Calculus and Alexander Ideals 59 7.1 TheFreeCalculus ............................... -

Maximum Knotted Switching Sequences
Maximum Knotted Switching Sequences Tom Milnor August 21, 2008 Abstract In this paper, we define the maximum knotted switching sequence length of a knot projection and investigate some of its properties. In par- ticular, we examine the effects of Reidemeister and flype moves on maxi- mum knotted switching sequences, show that every knot has projections having a full sequence, and show that all minimal alternating projections of a knot have the same length maximum knotted switching sequence. Contents 1 Introduction 2 1.1 Basic definitions . 2 1.2 Changing knot projections . 3 2 Maximum knotted switching sequences 4 2.1 Immediate bounds . 4 2.2 Not all maximal knotted switching sequences are maximum . 5 2.3 Composite knots . 5 2.4 All knots have a projection with a full sequence . 6 2.5 An infinite family of knots with projections not having full se- quences . 6 3 The effects of Reidemeister moves on the maximum knotted switching sequence length of a knot projection 7 3.1 Type I Reidemeister moves . 7 3.2 Type II Reidemeister moves . 8 3.3 Alternating knots and Type II Reidemeister moves . 9 3.4 The Conway polynomial and Type II Reidemeister moves . 13 3.5 Type III Reidemeister moves . 15 4 The maximum sequence length of minimal alternating knot pro- jections 16 4.1 Flype moves . 16 1 1 Introduction 1.1 Basic definitions A knot is a smooth embedding of S1 into R3 or S3. A knot is said to be an unknot if it is isotopic to S1 ⊂ R3. Otherwise it is knotted. -

Knot and Link Tricolorability Danielle Brushaber Mckenzie Hennen Molly Petersen Faculty Mentor: Carolyn Otto University of Wisconsin-Eau Claire
Knot and Link Tricolorability Danielle Brushaber McKenzie Hennen Molly Petersen Faculty Mentor: Carolyn Otto University of Wisconsin-Eau Claire Problem & Importance Colorability Tables of Characteristics Theorem: For WH 51 with n twists, WH 5 is tricolorable when Knot Theory, a field of Topology, can be used to model Original Knot The unknot is not tricolorable, therefore anything that is tri- 1 colorable cannot be the unknot. The prime factors of the and understand how enzymes (called topoisomerases) work n = 3k + 1 where k ∈ N ∪ {0}. in DNA processes to untangle or repair strands of DNA. In a determinant of the knot or link provides the colorability. For human cell nucleus, the DNA is linear, so the knots can slip off example, if a knot’s determinant is 21, it is 3-colorable (tricol- orable), and 7-colorable. This is known by the theorem that Proof the end, and it is difficult to recognize what the enzymes do. Consider WH 5 where n is the number of the determinant of a knot is 0 mod n if and only if the knot 1 However, the DNA in mitochon- full positive twists. n is n-colorable. dria is circular, along with prokary- Link Colorability Det(L) Unknot/Link (n = 1...n = k) otic cells (bacteria), so the enzyme L Number Theorem: If det(L) = 0, then L is WOLOG, let WH 51 be colored in this way, processes are more noticeable in 3 3 3 1 n 1 n-colorable for all n. excluding coloring the twist component. knots in this type of DNA. -

How Knot Theory Is Important to DNA Biology
How Knot Theory is important to DNA Biology PRIMES Circle 2020 William Ayinon About Me William Ayinon I am a sophomore at Newton North High School Interested in Computer Programming, Math, Biology and Chemistry Knot Theory ❖ The study of mathematical knots ❖ Theoretical string, ends glued together Knot Theory Terms ❏ Unknot - The simplest knot, it appears as a circle ❏ Deformation - A change to a knot that does not cut the string or pass it through itself somehow ❏ Invariant - A value that does not change when a knot is deformed Reidemeister Moves The Unknot Reidemeister Moves Crossings and Values Writhe: 1 Unknotting Number: 0 Over Under Writhe: 0 Unknotting Number: 0 ➔ ➔ Writhe - Total over crossings Unknotting number - Number of minus the total under crossings that need to be reversed in crossings order to form the unknot. Invariant DNA Biology Terms ❏ DNA - Deoxyribonucleic acid, a molecule responsible for every biological function within an organism ❏ Supercoiling - When DNA is coiled so tightly that it compresses itself like a telephone cord ❏ Enzyme - Proteins created by living organisms that bring about specific chemical reactions or chemical changes DNA in a cell Supercoiled DNA in a cell Topoisomerase Enzyme Knot Theory Applications ● Invariants / Knot Values ● DNA Knot Complexity ● How can we study the enzymes’ work? Cozzarelli and Brown E. Coli Studies ● Gyrase enzyme ● Rate of work by Gyrase Benefits Helps understand how to better manipulate DNA Developing field Studying how enzymes replicate DNA Cancer drugs try to prevent cell division Thank you for the support! ★ My parents ★ Peter Haine, mentor and coordinator ★ Kenneth Cox, mentor ★ PRIMES Circle as a whole Thank You Sources ● Adams, Colin C. -

Trefoil Knot, and Figure of Eight Knot
KNOTS, MOLECULES AND STICK NUMBERS TAKE A PIECE OF STRING. Tie a knot in it, and then glue the two loose ends of the string together. You have now trapped the knot on the string. No matter how long you attempt to disentangle the string, you will never succeed without cutting the string open. INEQUIVALENT KNOTS THE TRIVIAL KNOT, TREFOIL KNOT, AND FIGURE OF EIGHT KNOT Thanks to COLIN ADAMS: plus.maths.org Here are some famous knots, all known to be INEQUIVALENT. In other words, none of these three can be rearranged to look like the others. However, proving this fact is difficult. This is where the math comes in. The simple loop of string on the top is the UNKNOT, also known as the trivial knot. The TREFOIL KNOT and the FIGURE-EIGHT KNOT are the two simplest nontrivial knots, the first having three crossings and the second, four. No other nontrivial knots can be drawn with so few crossings. DISTINCT KNOTS Over the years, mathematicians have created tables of knots, all known to be distinct. SOME DISTINCT 10-CROSSING KNOTS So far, over 1.7 million inequivalent knots with pictures of 16 or fewer crossings have been identified. KNOTS, MOLECULES AND STICK NUMBERS THE matHEmatical THEORY OF KNOTS WAS BORN OUT OF attEMPTS TO MODEL THE atom. Near the end of the nineteenth century, Lord Kelvin suggested that different atoms were actually different knots tied in the ether that was believed to permeate all of space. Physicists and mathematicians set to work making a table of distinct knots, believing they were making a table of the elements. -

Petal Projections, Knot Colorings and Determinants
PETAL PROJECTIONS, KNOT COLORINGS & DETERMINANTS ALLISON HENRICH AND ROBIN TRUAX Abstract. An ¨ubercrossing diagram is a knot diagram with only one crossing that may involve more than two strands of the knot. Such a diagram without any nested loops is called a petal projection. Every knot has a petal projection from which the knot can be recovered using a permutation that represents strand heights. Using this permutation, we give an algorithm that determines the p-colorability and the determinants of knots from their petal projections. In particular, we compute the determinants of all prime knots with crossing number less than 10 from their petal permutations. 1. Background There are many different ways to define a knot. The standard definition of a knot, which can be found in [1] or [4], is an embedding of a closed curve in three-dimensional space, K : S1 ! R3. Less formally, we can think of a knot as a knotted circle in 3-space. Though knots exist in three dimensions, we often picture them via 2-dimensional representations called knot diagrams. In a knot diagram, crossings involve two strands of the knot, an overstrand and an understrand. The relative height of the two strands at a crossing is conveyed by putting a small break in one strand (to indicate the understrand) while the other strand continues over the crossing, unbroken (this is the overstrand). Figure 1 shows an example of this. Two knots are defined to be equivalent if one can be continuously deformed (without passing through itself) into the other. In terms of diagrams, two knot diagrams represent equivalent knots if and only if they can be related by a sequence of Reidemeister moves and planar isotopies (i.e. -

An Introduction to Knot Theory
An Introduction to Knot Theory Matt Skerritt (c9903032) June 27, 2003 1 Introduction A knot, mathematically speaking, is a closed curve sitting in three dimensional space that does not intersect itself. Intuitively if we were to take a piece of string, cord, or the like, tie a knot in it and then glue the loose ends together, we would have a knot. It should be impossible to untangle the knot without cutting the string somewhere. The use of the word `should' here is quite deliberate, however. It is possible, if we have not tangled the string very well, that we will be able to untangle the mess we created and end up with just a circle of string. Of course, this circle of string still ¯ts the de¯nition of a closed curve sitting in three dimensional space and so is still a knot, but it's not very interesting. We call such a knot the trivial knot or the unknot. Knots come in many shapes and sizes from small and simple like the unknot through to large and tangled and messy, and beyond (and everything in between). The biggest questions to a knot theorist are \are these two knots the same or di®erent" or even more importantly \is there an easy way to tell if two knots are the same or di®erent". This is the heart of knot theory. Merely looking at two tangled messes is almost never su±cient to tell them apart, at least not in any interesting cases. Consider the following four images for example. -
![Arxiv:2104.14076V1 [Math.GT] 29 Apr 2021 Gauss Codes for Each of the Unknot Diagrams Discussed in This Note in Ap- Pendixa](https://docslib.b-cdn.net/cover/9961/arxiv-2104-14076v1-math-gt-29-apr-2021-gauss-codes-for-each-of-the-unknot-diagrams-discussed-in-this-note-in-ap-pendixa-2039961.webp)
Arxiv:2104.14076V1 [Math.GT] 29 Apr 2021 Gauss Codes for Each of the Unknot Diagrams Discussed in This Note in Ap- Pendixa
HARD DIAGRAMS OF THE UNKNOT BENJAMIN A. BURTON, HSIEN-CHIH CHANG, MAARTEN LÖFFLER, ARNAUD DE MESMAY, CLÉMENT MARIA, SAUL SCHLEIMER, ERIC SEDGWICK, AND JONATHAN SPREER Abstract. We present three “hard” diagrams of the unknot. They re- quire (at least) three extra crossings before they can be simplified to the 2 trivial unknot diagram via Reidemeister moves in S . Both examples are constructed by applying previously proposed methods. The proof of their hardness uses significant computational resources. We also de- termine that no small “standard” example of a hard unknot diagram 2 requires more than one extra crossing for Reidemeister moves in S . 1. Introduction Hard diagrams of the unknot are closely connected to the complexity of the unknot recognition problem. Indeed a natural approach to solve instances of the unknot recognition problem is to untangle a given unknot diagram by Reidemeister moves. Complicated diagrams may require an exhaustive search in the Reidemeister graph that very quickly becomes infeasible. As a result, such diagrams are the topic of numerous publications [5, 6, 11, 12, 15], as well as discussions by and resources provided by leading researchers in low-dimensional topology [1,3,7, 14]. However, most classical examples of hard diagrams of the unknot are, in fact, easy to handle in practice. Moreover, constructions of more difficult examples often do not come with a rigorous proof of their hardness [5, 12]. The purpose of this note is hence two-fold. Firstly, we provide three un- knot diagrams together with a proof that they are significantly more difficult to untangle than classical examples.