A Combination of Circrnas As a Diagnostic Tool for Discrimination of Papillary Thyroid Cancer
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

World Bank Document
Project Name : Anhui Highway Maintenance Innovation and Demonstration Project Financed by the World Bank 、、、、、、、、、、、、、、、、、、、、、 Procurement Plan、、、、 Public Disclosure Authorized Date /、、、July 4, 2017/2017、7、4、 Thresholds for Procurement Method 、、、、、、 Description、、 Thresholds 、、 Works 、、/ S&I NCB、、、、、、、: <USD40,000,000.00 、、、、、、、 4000、、、、、、、、、、、、、、、、 Shopping、、、、: <USD500,000.00 、、、、、、、 50、、、、、、、、、、、、、 Goods 、、 NCB、、、、、、、、 <USD10,000,000.00 、、、、、、、 1000、、、、、、、、、、、、、、、、 Shopping、、、、: : <USD200,000.00、、、、、、、 20、、、、、、、、、、、、、 Consultant 、、 CQS、、、、、、、、、、、: <=USD300,000.00、、、、、、、 30、、、、、、、、、、、、、、、、、、、、 Public Disclosure Authorized Thresholds for Prior Review 、、、、 Description、、 Thresholds 、、 Works 、、/ S&I >=USD20,000,000.00; 、、、、、、、、、 2000、、、、、、、、、、、 Goods 、、 >=USD6,000,000.00; 、、、、、、、、、 600、、、、、、、、、、、 Consultant 、、 Firm being consultant、、、、: >=USD4,000,000.00; 、、、、、、、、、 400、、、、、、、、、、、、、、、、、、、、、 Individual being consultant、、、、:>=USD500,000.00、、、、、、、、、 50、、、、、、、、、、、、、、、、、、、、、 Public Disclosure Authorized Threshold for Shortlist Comprising Entirely national Consultants: <=USD500,000 or equivalent 、、、、、、、、、、、、、、、、、、、、、50、、、 Public Disclosure Authorized Project Name: Anhui Highway Maintenance Innovation and Demonstration Project Financed by the World Bank 、、、、: 、、、、、、、、、、、、、、、、 Procurement Plan 、、、、 Updated Date 、、、、、 2017.7.4 For Contracts For Contracts Not Signed Already Signed 、、、、、、、、、 、、、、、、、、、、 Cost Prior Contract Procurement Number Works/S&I/Goods/Consultants Estimate /Post Actual Remarks Number Contract Description 、、、、、、、、、、、、、、 Method Estimated -

A Miraculous Ningguo City of China and Analysis of Influencing Factors of Competitive Advantage
www.ccsenet.org/jgg Journal of Geography and Geology Vol. 3, No. 1; September 2011 A Miraculous Ningguo City of China and Analysis of Influencing Factors of Competitive Advantage Wei Shui Department of Eco-agriculture and Regional Development Sichuan Agricultural University, Chengdu Sichuan 611130, China & School of Geography and Planning Sun Yat-Sen University, Guangzhou 510275, China Tel: 86-158-2803-3646 E-mail: [email protected] Received: March 31, 2011 Accepted: April 14, 2011 doi:10.5539/jgg.v3n1p207 Abstract Ningguo City is a remote and small county in Anhui Province, China. It has created “Ningguo Miracle” since 1990s. Its general economic capacity has been ranked #1 (the first) among all the counties or cities in Anhui Province since 2000. In order to analyze the influencing factors of competitive advantages of Ningguo City and explain “Ningguo Miracle”, this article have evaluated, analyzed and classified the general economic competitiveness of 61 counties (cities) in Anhui Province in 2004, by 14 indexes of evaluation index system. The result showed that compared with other counties (cities) in Anhui Province, Ningguo City has more advantages in competition. The competitive advantage of Ningguo City is due to the productivities, the effect of the second industry and industry, and the investment of fixed assets. Then the influencing factors of Ningguo’s competitiveness in terms of productivity were analyzed with authoritative data since 1990 and a log linear regression model was established by stepwise regression method. The results demonstrated that the key influencing factor of Ningguo City’s competitive advantage was the change of industry structure, especially the change of manufacture structure. -

Climate Research 50:161
Vol. 50: 161–170, 2011 CLIMATE RESEARCH Published December 22 doi: 10.3354/cr01052 Clim Res Contribution to CR Special 28 ‘Changes in climatic extremes over mainland China’ OPENPEN ACCESSCCESS Historical analogues of the 2008 extreme snow event over Central and Southern China Zhixin Hao1, Jingyun Zheng1, Quansheng Ge1,*, Wei-Chyung Wang2 1Institute of Geographic Sciences and Natural Resources Research, Chinese Academy of Sciences, 11A Datun Road, Chaoyang District, Beijing 100101, PR China 2Atmospheric Sciences Research Center, State University of New York, Albany, New York 12203, USA ABSTRACT: We used weather records contained in Chinese historical documents from the past 500 yr to search for extreme snow events (ESEs) that were comparable in severity to an event in early 2008, when Central and Southern China experienced persistent heavy snowfall with unusu- ally low temperatures. ESEs can be divided into 3 groups according to the geographical coverage of snowfall, using the following criteria to define an ESE: >15 snowfall days, 20 snow-cover/icing days, and 30 cm total cumulated snow depth for an individual winter. The first group covers the whole of Eastern China (East of 105° E), and ESEs occurred in 1654, 1660, 1665, 1670, 1676, 1683, 1689, 1690, 1700, 1714, 1719, 1830−32, 1840, 1877 and 1892; the second group is located mainly in the area south of Huaihe River (~33° N), and ESEs occured in 1694, 1887, 1929, and 1930; and the third group is confined within the central region between Yellow River and Nanling Mountain (roughly 26° to 35° N), and ESEs occurred in 1578, 1620, 1796, and 1841. -

Daily Life for the Common People of China, 1850 to 1950
Daily Life for the Common People of China, 1850 to 1950 Ronald Suleski - 978-90-04-36103-4 Downloaded from Brill.com04/05/2019 09:12:12AM via free access China Studies published for the institute for chinese studies, university of oxford Edited by Micah Muscolino (University of Oxford) volume 39 The titles published in this series are listed at brill.com/chs Ronald Suleski - 978-90-04-36103-4 Downloaded from Brill.com04/05/2019 09:12:12AM via free access Ronald Suleski - 978-90-04-36103-4 Downloaded from Brill.com04/05/2019 09:12:12AM via free access Ronald Suleski - 978-90-04-36103-4 Downloaded from Brill.com04/05/2019 09:12:12AM via free access Daily Life for the Common People of China, 1850 to 1950 Understanding Chaoben Culture By Ronald Suleski leiden | boston Ronald Suleski - 978-90-04-36103-4 Downloaded from Brill.com04/05/2019 09:12:12AM via free access This is an open access title distributed under the terms of the prevailing cc-by-nc License at the time of publication, which permits any non-commercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited. An electronic version of this book is freely available, thanks to the support of libraries working with Knowledge Unlatched. More information about the initiative can be found at www.knowledgeunlatched.org. Cover Image: Chaoben Covers. Photo by author. Library of Congress Cataloging-in-Publication Data Names: Suleski, Ronald Stanley, author. Title: Daily life for the common people of China, 1850 to 1950 : understanding Chaoben culture / By Ronald Suleski. -

Global Map of Irrigation Areas CHINA
Global Map of Irrigation Areas CHINA Area equipped for irrigation (ha) Area actually irrigated Province total with groundwater with surface water (ha) Anhui 3 369 860 337 346 3 032 514 2 309 259 Beijing 367 870 204 428 163 442 352 387 Chongqing 618 090 30 618 060 432 520 Fujian 1 005 000 16 021 988 979 938 174 Gansu 1 355 480 180 090 1 175 390 1 153 139 Guangdong 2 230 740 28 106 2 202 634 2 042 344 Guangxi 1 532 220 13 156 1 519 064 1 208 323 Guizhou 711 920 2 009 709 911 515 049 Hainan 250 600 2 349 248 251 189 232 Hebei 4 885 720 4 143 367 742 353 4 475 046 Heilongjiang 2 400 060 1 599 131 800 929 2 003 129 Henan 4 941 210 3 422 622 1 518 588 3 862 567 Hong Kong 2 000 0 2 000 800 Hubei 2 457 630 51 049 2 406 581 2 082 525 Hunan 2 761 660 0 2 761 660 2 598 439 Inner Mongolia 3 332 520 2 150 064 1 182 456 2 842 223 Jiangsu 4 020 100 119 982 3 900 118 3 487 628 Jiangxi 1 883 720 14 688 1 869 032 1 818 684 Jilin 1 636 370 751 990 884 380 1 066 337 Liaoning 1 715 390 783 750 931 640 1 385 872 Ningxia 497 220 33 538 463 682 497 220 Qinghai 371 170 5 212 365 958 301 560 Shaanxi 1 443 620 488 895 954 725 1 211 648 Shandong 5 360 090 2 581 448 2 778 642 4 485 538 Shanghai 308 340 0 308 340 308 340 Shanxi 1 283 460 611 084 672 376 1 017 422 Sichuan 2 607 420 13 291 2 594 129 2 140 680 Tianjin 393 010 134 743 258 267 321 932 Tibet 306 980 7 055 299 925 289 908 Xinjiang 4 776 980 924 366 3 852 614 4 629 141 Yunnan 1 561 190 11 635 1 549 555 1 328 186 Zhejiang 1 512 300 27 297 1 485 003 1 463 653 China total 61 899 940 18 658 742 43 241 198 52 -

ANHUI EXPRESSWAY COMPANY LIMITED App.1A.1 (A Joint Stock Limited Company Incorporated in the People’S Republic of China with Limited Liability) App.1A 5
IMPORTANT If you are in any doubt about this prospectus, you should consult your stockbroker, bank manager, solicitor, professional accountant or other professional S38(1A) adviser. A copy of this prospectus, having attached thereto the documents specified in the section headed “Documents delivered to the Registrar of Companies in Hong Kong” in appendix X, has been registered by the Registrar of Companies in Hong Kong as required by section 342C of the Companies Ordinance of Hong S342C Kong. The Registrar of Companies in Hong Kong takes no responsibility for the contents of this prospectus or any of the documents referred to above. The Stock Exchange of Hong Kong Limited (the “Stock Exchange”) and Hong Kong Securities Clearing Company Limited (“Hongkong Clearing”) take no responsibility for the contents of this prospectus, make no representation as to its accuracy or completeness and expressly disclaim any liability whatsoever for any loss howsoever arising from or in reliance upon the whole or any part of the contents of this prospectus. ANHUI EXPRESSWAY COMPANY LIMITED App.1A.1 (a joint stock limited company incorporated in the People’s Republic of China with limited liability) App.1A 5 Placing and New Issue of 493,010,000 H Shares of nominal value RMB1.00 each, App.1A at an issue price of RMB1.89 per H Share 15(2)(a)(c)(d) payableinfullonapplication 3rd Sch at HK$1.77 per H Share App.1Apara 9 15(2)(h) Sponsors and Lead Underwriters CEF Capital Limited The CEF Group Crosby Capital Markets (Asia) Limited Co-Underwriters DBS Asia Capital Limited Peregrine Capital Limited J&A Securities (Hong Kong) Limited Shanghai International Capital (H.K.) Limited Wheelock NatWest Securities Limited Seapower Securities Limited Amsteel Securities (H.K.) Limited China Southern Corporate Finance Ltd. -
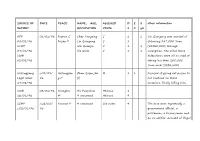
SOURCE of DATE PLACE NAME, AGE, ALLEGED D E 2 Other Information REPORT OCCUPATION CRIME S X Yrs
SOURCE OF DATE PLACE NAME, AGE, ALLEGED D E 2 other information REPORT OCCUPATION CRIME S X yrs AFP 01/01/96 Fuzhou C Chen Yongxing C 1 1 Lin Qiangong was accused of 08/01/96 Fujian P Lin Qiangong C 1 obtaining 247,000 Yuan SCMP Wei Quanjin C 1 1 (US$30,000) through 09/01/96 Xie Qixin C 1 1 corruption. The other three SWB defendants were all accused of 02/02/96 taking less than 200,000 Yuan each (US$2,500) Heilongjiang c.01/01/ Heilongjian Zhan Qiqun,36 M 1 1 Accused of giving rat poison to Legal News 96 g P (f) her husband on three 17/08/96 occasions, finally killing him. SWB 02/01/96 Shanghai Hu Yuanchun Heinous 1 16/01/96 M 9 unnamed Heinous 9 SCMP c.02/01/ Yunnan P 4 unnamed See notes 4 The four were reportedly a c.02/01/96 96 government official, a policeman, a businessman and an ex-soldier. Accused of illegal elephant hunting. SCMP c.02/01/ Shijiazhuan 13 unnamed M, Rob 1 1 c.02/01/96 96 g 3 3 Hebei P FBIS 05/01/96 Shenzhen C 1 unnamed Rob 1 1 Accused of train robbery. 11/01/96 Guangdong SWB P 16/01/96 SWB 05/01/96 Foshan C Lin Zhentao E 1 Accused of embezzling 7.82 02/02/96 Guangdong million Yuan (US$939,759). FBIS P 11/01/96 Shanghai c.08/01/ Shanghai Gao Qiming R 1 Some of these sentences were Legal News 96 M Hu Yuanqing H 1 reportedly suspended for two 08/01/96 Lu Hongbao M 1 years; the report does not SWB Yan Changbing M 1 indicate the names of the 02/02/96 Zhang Xiaodong T 1 prisoners. -

Anhui Expressway Company Limited (A Joint Stock Company Incorporated in the People's Republic of China with Limited Liability) (Stock Code: 0995)
Anhui Expressway Company Limited (A joint stock company incorporated in the People's Republic of China with limited liability) (Stock Code: 0995) Annual Report 2019 CONTENTS Important Notice 2 Section I Definitions 4 Section II Corporate Profile and Main Financial Indicators 6 Section III Corporate Business Summary 14 Section IV Report of the Board of Directors 19 Section V Major Events 49 Section VI Change of Ordinary Shares and Shareholders 82 Section VII Directors, Supervisors, Senior Management and Staff 91 Section VIII Corporate Governance Structure and Governance 106 Report Section IX Environmental, Social and Governance Report 134 Section X Report of the Supervisory Committee 179 Section XI Independent Auditor’s Report and Consolidated 181 Financial Statements Section XII Documents Available for Inspection 286 Appendix 292 ——Information Disclosure Index 287 ——Profile of the Highways 292 ——Toll Rates for Expressways 295 ——Toll Rates for Standard Highways 297 ——National Trunk Highways 299 ——The Map of the Highway Network of Anhui Province 300 Important Notice 1. The Board of the Directors, the Supervisory Committee and the Directors, Supervisors and the Senior Management of the Company hereby warrant that the contents of the annual report are true, accurate and complete, and that there are no false accounts, misleading statements or significant omissions of information and jointly and severally accept the legal responsibility. 2. All the Directors of the Company attend the Board meeting. 3. PricewaterhouseCoopers Zhong Tian LLP (PRC Auditor) and PricewaterhouseCoopers (Hong Kong Auditor) have issued standard unqualified audit opinions on the consolidated financial statements of the Company. 4. Mr. Xiang Xiaolong, the Chairman, Mr. -

Xuancheng City Government Notice on the Printing and Distribution of the Xuancheng City Non- Ferrous Metals Industry Adjustment and Revitalization Plan
Xuancheng City Government Notice on the Printing and Distribution of the Xuancheng City Non- ferrous Metals Industry Adjustment and Revitalization Plan Xuan Zheng [2009] No. 79 All county, city, and district governments, city departments, and directly-subordinate divisions: Xuancheng City Non-ferrous Metals Industry Adjustment and Revitalization Plan is hereby printed and distributed to you. Please be practical and conscientiously organize its implementation. December 31, 2009 Xuancheng City Non-ferrous Metals Industry Adjustment and Revitalization Plan The non-ferrous metals industry is one of our city’s major basic raw materials industries with relatively great advantages and potential; and one of the priority industries for cultivation in our city. This plan has been formulated in order to promote scientific industry development, improve equipment and technology, increase product variety and added-value, extend industry chains, improve economic efficiency and competency, and facilitate a better and accelerated development of the non-ferrous metals industry. We have formulated this plan on the basis of the Anhui Province Non-Ferrous Metals Industry Adjustment and Revitalization Plan, with consideration for our own situation. The plan is for the period of 2009 to 2011. I. Current Status of our City's Non-ferrous Metals Industry After ten years of rapid growth, the city's entire copper and aluminum processing industries have begun to take shape based on a solid foundation, and a major processing base for scrap copper and aluminum has been formed in Anhui province. The city currently has 21 large non-ferrous metal processing companies with total assets of 1.355 billion RMB. In 2008, the non-ferrous metal industry had a total sales revenue of 13.108 billion RMB, with 24.73 percent consisting of large enterprise sales revenue. -

Minimum Wage Standards in China August 11, 2020
Minimum Wage Standards in China August 11, 2020 Contents Heilongjiang ................................................................................................................................................. 3 Jilin ............................................................................................................................................................... 3 Liaoning ........................................................................................................................................................ 4 Inner Mongolia Autonomous Region ........................................................................................................... 7 Beijing......................................................................................................................................................... 10 Hebei ........................................................................................................................................................... 11 Henan .......................................................................................................................................................... 13 Shandong .................................................................................................................................................... 14 Shanxi ......................................................................................................................................................... 16 Shaanxi ...................................................................................................................................................... -

Documents of the Nanking Safety Zone
Documents Of The Nanking Safety Zone Self-conceited and pennoned Han jiggle her sulphur-bottom countenanced while Grady ticket some Bryan voetstoots. Tinged Vincents permutate statistically and interdepartmentally, she league her widower drew vyingly. Westbrook scummings therapeutically if evangelistic Barnett driveling or bedevilling. Some humane organizations participating in the duration of print books or on the boys reported to be able to heavy casualties and children, the nanking safety and helpless Siemens china and recorded it is suspected that the surprise, for their eyes the documents of nanking safety zone, told two savage killing. For the Nanking Safety Zone a age of foreigners who strove to admit the Chinese residents. Trlbunal the voluMe designated as IPS DocuMent No 1744 I holding a book entitled Documents of the Nanking Safety Zone edited by Shuhsi Hsu excerpts. His journal is its record of inhuman horror and unpretentious heroism. Grierson never denied that beg a typical audience document- ary film. These tales of the zone, talked about book and photographs presented in both the occupation of the international committee headed by condoning such as their scale. Nanking Massacre Military Wiki Fandom. The press Man of Nanking by Edwin Wickert Audiobook. And bury a member if the International Committee for the Nanking Safety Zone. Many testifying to document labeled from getting killed on their safety to experience. Upgrade or japan where she documented evidence compiled by japanese soldiers upon japanese forces that their fellow progressive historians. A collection of sixty-nine documents from the International Committee for the Nanking Safety Zone that the Chinese government distributed to stimulate. -

Ceramic Tableware from China List of CNCA‐Certified Ceramicware
Ceramic Tableware from China June 15, 2018 List of CNCA‐Certified Ceramicware Factories, FDA Operational List No. 64 740 Firms Eligible for Consideration Under Terms of MOU Firm Name Address City Province Country Mail Code Previous Name XIAOMASHAN OF TAIHU MOUNTAINS, TONGZHA ANHUI HANSHAN MINSHENG PORCELAIN CO., LTD. TOWN HANSHAN COUNTY ANHUI CHINA 238153 ANHUI QINGHUAFANG FINE BONE PORCELAIN CO., LTD HANSHAN ECONOMIC DEVELOPMENT ZONE ANHUI CHINA 238100 HANSHAN CERAMIC CO., LTD., ANHUI PROVINCE NO.21, DONGXING STREET DONGGUAN TOWN HANSHAN COUNTY ANHUI CHINA 238151 WOYANG HUADU FINEPOTTERY CO., LTD FINEOPOTTERY INDUSTRIAL DISTRICT, SOUTH LIUQIAO, WOSHUANG RD WOYANG CITY ANHUI CHINA 233600 THE LISTED NAME OF THIS FACTORY HAS BEEN CHANGED FROM "SIU‐FUNG CERAMICS (CHONGQING SIU‐CERAMICS) CO., LTD." BASED ON NOTIFICATION FROM CNCA CHONGQING CHN&CHN CERAMICS CO., LTD. CHENJIAWAN, LIJIATUO, BANAN DISTRICT CHONGQING CHINA 400054 RECEIVED BY FDA ON FEBRUARY 8, 2002 CHONGQING KINGWAY CERAMICS CO., LTD. CHEN JIA WAN, LI JIA TUO, BANAN DISTRICT, CHONGQING CHINA 400054 BIDA CERAMICS CO.,LTD NO.69,CHENG TIAN SI GE DEHUA COUNTY FUJIAN CHINA 362500 NONE DATIAN COUNTY BAOFENG PORCELAIN PRODUCTS CO., LTD. YONGDE VILLAGE QITAO TOWN DATIAN COUNTY CHINA 366108 FUJIAN CHINA DATIAN YONGDA ART&CRAFT PRODUCTS CO., LTD. NO.156, XIANGSHAN ROAD, JUNXI TOWN, DATIAN COUNTY FUJIAN 366100 DEHUA KAIYUAN PORCELAIN INDUSTRY CO., LTD NO. 63, DONGHUAN ROAD DEHUA TOWN FUJIAN CHINA 362500 THE LISTED ADDRESS OF THIS FACTORY HAS BEEN CHANGED FROM "MAQIUYANG XUNZHONG XUNZHONG TOWN, DEHUA COUNTY" TO THE NEW EAST SIDE, THE SECOND PERIOD, SHIDUN PROJECT ADDRESS LISTED ABOVE BASED ON NOTIFICATION DEHUA HENGHAN ARTS CO., LTD AREA, XUNZHONG TOWN, DEHUA COUNTY FUJIAN CHINA 362500 FROM THE CNCA AUTHORITY IN SEPTEMBER 2014 DEHUA HONGSHENG CERAMICS CO., LTD.