Articles, However, Are Largely Unknown
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Clay Minerals Soils to Engineering Technology to Cat Litter
Clay Minerals Soils to Engineering Technology to Cat Litter USC Mineralogy Geol 215a (Anderson) Clay Minerals Clay minerals likely are the most utilized minerals … not just as the soils that grow plants for foods and garment, but a great range of applications, including oil absorbants, iron casting, animal feeds, pottery, china, pharmaceuticals, drilling fluids, waste water treatment, food preparation, paint, and … yes, cat litter! Bentonite workings, WY Clay Minerals There are three main groups of clay minerals: Kaolinite - also includes dickite and nacrite; formed by the decomposition of orthoclase feldspar (e.g. in granite); kaolin is the principal constituent in china clay. Illite - also includes glauconite (a green clay sand) and are the commonest clay minerals; formed by the decomposition of some micas and feldspars; predominant in marine clays and shales. Smectites or montmorillonites - also includes bentonite and vermiculite; formed by the alteration of mafic igneous rocks rich in Ca and Mg; weak linkage by cations (e.g. Na+, Ca++) results in high swelling/shrinking potential Clay Minerals are Phyllosilicates All have layers of Si tetrahedra SEM view of clay and layers of Al, Fe, Mg octahedra, similar to gibbsite or brucite Clay Minerals The kaolinite clays are 1:1 phyllosilicates The montmorillonite and illite clays are 2:1 phyllosilicates 1:1 and 2:1 Clay Minerals Marine Clays Clays mostly form on land but are often transported to the oceans, covering vast regions. Kaolinite Al2Si2O5(OH)2 Kaolinite clays have long been used in the ceramic industry, especially in fine porcelains, because they can be easily molded, have a fine texture, and are white when fired. -

Halloysite Formation Through in Situ Weathering of Volcanic Glass From
Ciay Minerals (1988) 23, 423-431 mineralogy. Phys. ition of Mössbauer L HALLOYSITE FORMATION THROUGH Ih' SITU shaviour? J. Mag. WEATHERING OF VOLCANIC GLASS FROM 3n analysis of two TRACHYTIC PUMICES, VICO'S VOLCANO, ITALY d.X, 29-31. ir Conímbriga and P. QUANTIN, J. GAUTHEYROU AND P. LORENZONI* ts correlation with .central Portugal). ORSTOM, 70 route d'Aulnay. 93143 Bondy Cedex, France, and *ISSDS, Piazza d'Azeglio 30, Firenze, Italy ibrico da cerâmica (Received October 1987; revised 5 April 1988) of dolomites, clays ABSTRACT: The weathering of a trachytic pumice within a pyroclastic flow underlying an ., MADWCKA.G., andic-brown soil on the volcano Vico has been studied. The main mineral formed is a spherical 10 A halloysite which has been shown by SEM and in situ microprobe analysis to have formed norphology on the directly from the glass. The major mineralogical characteristics as determined by XRD, IR, DTA, TEM and microdiffraction are typical of 10 A halloysite. However, some minor mineralogical properties and the high Fe and K contents, suggest that it is an interstratification of 74% halloysite and 26% illite-smectite. The calculated formula of the hypothetical 2:l minerals reveals an Fe- and K-rich clay, with high tetrahedral substitution, like an Fe-rich vermiculite, but the detailed structure of this mineral remains uncertain. This study deals with the weathering of trachytic pumices to a white clay which seems to be derived directly from glass, without change in texture. This clay is a well crystallized 10 A halloysite, and although nearly white in colour, has an unusual composition being rich in Fe and Ti, and having a high K content. -

Mixed-Layer Illite-Smectite in Pennsylvanian-Aged Paleosols: Assessing Sources of Illitization in the Illinois Basin
minerals Article Mixed-Layer Illite-Smectite in Pennsylvanian-Aged Paleosols: Assessing Sources of Illitization in the Illinois Basin Julia A. McIntosh 1,* , Neil J. Tabor 1 and Nicholas A. Rosenau 2 1 Roy M. Huffington Department of Earth Sciences, Southern Methodist University, P.O. Box 750395, Dallas, TX 75275, USA; [email protected] 2 Ocean and Coastal Management Branch, Office of Wetlands Oceans and Watersheds, United States Environmental Protection Agency, Washington, DC 20004, USA; [email protected] * Correspondence: [email protected]; Tel.: +1-214-768-2750 Abstract: Mixed-layer illite-smectite (I-S) from a new set of Pennsylvanian-aged Illinois Basin under- clays, identified as paleosols, are investigated to assess the impact of (1) regional diagenesis across the basin and (2) the extent to which ancient environments promoted illitization during episodes of soil formation. Interpretations from Reichweite Ordering and D◦ 2q metrics applied to X-ray diffraction patterns suggest that most I-S in Illinois Basin paleosols are likely the product of burial diagenetic processes and not ancient soil formation processes. Acid leaching from abundant coal units and hydrothermal brines are likely diagenetic mechanisms that may have impacted I-S in Pennsylvanian paleosols. These findings also suggest that shallowly buried basins (<3 km) such as the Illinois Basin may still promote clay mineral alteration through illitization pathways if maximum burial occurred in the deep past and remained within the diagenetic window for extended periods of time. More importantly, since many pedogenic clay minerals may have been geochemically reset during illitization, sources of diagenetic alteration in the Illinois Basin should be better understood if Citation: McIntosh, J.A.; Tabor, N.J.; Pennsylvanian paleosol minerals are to be utilized for paleoclimate reconstructions. -

Illite Springer-Verlag Berlin Heidelberg Gmbh Alain Meunier • Bruce Velde
Illite Springer-Verlag Berlin Heidelberg GmbH Alain Meunier • Bruce Velde Illite Origins, Evolution and Metamorphism With 138 Figures Springer Professor Dr. Alain Meunier University of Poitiers UMR 6532 CNRS HYDRASA Laboratory 40, Avenue du Recteur Pineau 86022 Poitiers, France E-mail [email protected] Dr. Bruce Velde Research Director CNRS Geology Laboratory UMR 8538 CNRS Ecole Normale Superieure 24, rue Lhomond 75231 Paris, France E-mail [email protected] ISBN 978-3-642-05806-6 ISBN 978-3-662-07850-1 (eBook) DOI 10.1007/978-3-662-07850-1 Library of Congress Control Number: '3502272 Bibliographic information published by Die Deutsche Bibliothek Die Deutsche Bibliothek lists this publication in die Deutsche Nationalbibliography; detailed bibliographic data is available in the Internet at <http://dnb.ddb.de>. This work is subject to copyright. All rights are reserved, whether the whole or part of this material is concerned, specifically the rights of translation, reprinting, reuse of illustrations, recitations, broadcasting, reproduction on microfilm or in any other way, and storage in data banks. Duplication of this publication or parts thereof is permitted only under the provisions of the German Copyright Law of September 9, 1965, in its current version, and permission for use must always be obtained from Springer-Verlag. Violations are liable for prosecution under the German Copyright Law. springeronline.com © Springer-Verlag Berlin Heidelberg 2004 Originally published by Springer-Verlag Berlin Heidelberg New York in 2004. Softcover reprint of the hardcover I st edition 2004 The use of general descriptive names, registered names, trademarks, etc. in this publication does not imply, even in the absence of a specific statement, that such names are exempt from the relevant protective laws and regulations and therefore free for general use. -

Clay Minerals
CLAY MINERALS CD. Barton United States Department of Agriculture Forest Service, Aiken, South Carolina, U.S.A. A.D. Karathanasis University of Kentucky, Lexington, Kentucky, U.S.A. INTRODUCTION of soil minerals is understandable. Notwithstanding, the prevalence of silicon and oxygen in the phyllosilicate structure is logical. The SiC>4 tetrahedron is the foundation Clay minerals refers to a group of hydrous aluminosili- 2 of all silicate structures. It consists of four O ~~ ions at the cates that predominate the clay-sized (<2 |xm) fraction of apices of a regular tetrahedron coordinated to one Si4+ at soils. These minerals are similar in chemical and structural the center (Fig. 1). An interlocking array of these composition to the primary minerals that originate from tetrahedral connected at three corners in the same plane the Earth's crust; however, transformations in the by shared oxygen anions forms a hexagonal network geometric arrangement of atoms and ions within their called the tetrahedral sheet (2). When external ions bond to structures occur due to weathering. Primary minerals form the tetrahedral sheet they are coordinated to one hydroxyl at elevated temperatures and pressures, and are usually and two oxygen anion groups. An aluminum, magnesium, derived from igneous or metamorphic rocks. Inside the or iron ion typically serves as the coordinating cation and Earth these minerals are relatively stable, but transform- is surrounded by six oxygen atoms or hydroxyl groups ations may occur once exposed to the ambient conditions resulting in an eight-sided building block termed an of the Earth's surface. Although some of the most resistant octohedron (Fig. -

Download PDF About Minerals Sorted by Mineral Group
MINERALS SORTED BY MINERAL GROUP Most minerals are chemically classified as native elements, sulfides, sulfates, oxides, silicates, carbonates, phosphates, halides, nitrates, tungstates, molybdates, arsenates, or vanadates. More information on and photographs of these minerals in Kentucky is available in the book “Rocks and Minerals of Kentucky” (Anderson, 1994). NATIVE ELEMENTS (DIAMOND, SULFUR, GOLD) Native elements are minerals composed of only one element, such as copper, sulfur, gold, silver, and diamond. They are not common in Kentucky, but are mentioned because of their appeal to collectors. DIAMOND Crystal system: isometric. Cleavage: perfect octahedral. Color: colorless, pale shades of yellow, orange, or blue. Hardness: 10. Specific gravity: 3.5. Uses: jewelry, saws, polishing equipment. Diamond, the hardest of any naturally formed mineral, is also highly refractive, causing light to be split into a spectrum of colors commonly called play of colors. Because of its high specific gravity, it is easily concentrated in alluvial gravels, where it can be mined. This is one of the main mining methods used in South Africa, where most of the world's diamonds originate. The source rock of diamonds is the igneous rock kimberlite, also referred to as diamond pipe. A nongem variety of diamond is called bort. Kentucky has kimberlites in Elliott County in eastern Kentucky and Crittenden and Livingston Counties in western Kentucky, but no diamonds have ever been discovered in or authenticated from these rocks. A diamond was found in Adair County, but it was determined to have been brought in from somewhere else. SULFUR Crystal system: orthorhombic. Fracture: uneven. Color: yellow. Hardness 1 to 2. -

Structural Variations in Illite and Chlorite in the Belt Supergroup Western Montana and Northern Idaho
University of Montana ScholarWorks at University of Montana Graduate Student Theses, Dissertations, & Professional Papers Graduate School 1991 Structural variations in illite and chlorite in the Belt Supergroup western Montana and northern Idaho Peter Ryan The University of Montana Follow this and additional works at: https://scholarworks.umt.edu/etd Let us know how access to this document benefits ou.y Recommended Citation Ryan, Peter, "Structural variations in illite and chlorite in the Belt Supergroup western Montana and northern Idaho" (1991). Graduate Student Theses, Dissertations, & Professional Papers. 7192. https://scholarworks.umt.edu/etd/7192 This Thesis is brought to you for free and open access by the Graduate School at ScholarWorks at University of Montana. It has been accepted for inclusion in Graduate Student Theses, Dissertations, & Professional Papers by an authorized administrator of ScholarWorks at University of Montana. For more information, please contact [email protected]. Maureen and Mike MANSFIELD LIBRARY Copying allowed as provided under provisions of the Fair Use Section of the U.S. COPYRIGHT LAW, 1976. Any copying for commercial purposes or financM gain may be undertaken only with the author’s written consent. MontanaUniversity of Reproduced with permission of the copyright owner. Further reproduction prohibited without permission. Reproduced with permission of the copyright owner. Further reproduction prohibited without permission. STRUCTURAL VARIATIONS IN IlilTE AND CHLORITE IN THE BELT SUPERGROUP, WESTERN MONTANA AND NORTHERN IDAHO by PETER RYAN A.B., Dartmouth College, 1988 Presented in partial fulfillment of the requirements for the degree of Master of Science UNIVERSITY OF MONTANA 1991 iners ïan. Graduate School Z,g,. -
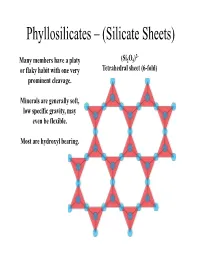
Phyllosilicates – (Silicate Sheets)
Phyllosilicates – (Silicate Sheets) 2- Many members have a platy (Si2O5) or flaky habit with one very Tetrahedral sheet (6-fold) prominent cleavage . Minerals are generally soft, low specific gravity, may even be flexible. Most are hydroxyl bearing. Each tetrahedra is bound to three neiggghboring tetrahedra via three basal bridging oxygens. The apical oxygen of each tetrahedral in a sheet all point in the same direction. The sheets are stacked either apice-to- apice or base-to-base. In an undistorted sheet the hydroxyl (OH) group sits in the centre and each outlined triangle is equivalent. Sheets within sheets…. Apical oxygens, plus the –OH group, coordinate a 6-fold (octahedral) site (XO6). These octahedral sites form infinitely extending sheets. All the octahedra lie on triangular faces, oblique to the tetrahedral sheets. The most common elements found in the 6 -fold site are Mg (or Fe) or Al . Dioctahedral vs Trioctahedral Mg and Al have different charges, but the sheet must remain charge neutral . With 6 coordinating oxygens, we have a partial charge of -6. How many Mg2+ ions are required to retain neutrality? How many Al3+ ions are required to retain neutrality? Mg occupies all octahedral sites, while Al will only occupy 2 out of every 3. The stacking of the sheets dictates the crystallography and c hem istry o f eac h o f t he p hhyll llosili cates. Trioctahedral Dioctahedral O Brucite Gibbsite Hydroxyl Magnesium Aluminium Trioctahedral Is this structure charge neutral? T O T Interlayer Cation T O T Potassium (K+) Phlogopite (Mg end-member biotite) Dioctahedral Is this structure charge neutral? T O T Interlayer Cation T O T Potassium (K+) Muscovite Compositional variation in phyllosilicates There is little solid solution between members of the dioctahedral and trioctahedral groups. -

Dispersion Characteristics of Montmorillonite, Kaolinite, and Hike Clays in Waters of Varying Quality, and Their Control with Phosphate Dispersants by B
Dispersion Characteristics of Montmorillonite, Kaolinite, and Hike Clays in Waters of Varying Quality, and Their Control with Phosphate Dispersants By B. N. ROLFE, R. F. MILLER, and I. S. McQUEEN SHORTER CONTRIBUTIONS TO GENERAL GEOLOGY GEOLOGICAL SURVEY PROFESSIONAL PAPER 334-G Prepared in cooperation with Colorado State University UNITED STATES GOVERNMENT PRINTING OFFICE, WASHINGTON : 1960 UNITED STATES DEPARTMENT OF THE INTERIOR FRED A. SEATON, Secretary GEOLOGICAL SURVEY Thomas B. Nolan, Director For sale by the Superintendent of Documents, U.S. Government Printing Office Washington 25, D.C. - Price 40 cents (paper cover) CONTENTS Page Results and discussion Continued Page Abstract._ ________________________________________ 229 Sodium illite in hard water_______________________ 241 Introduction.______________________________________ 229 Calcium illite in hard water._____________________ 243 Personnel._____________________________________ 230 Volclay in distilled water._______________________ 243 Acknowledgments _______________________________ 230 Volclay in medium-hard water- _-_-_-_---_.___ 243 Materials----------------------_---__---_----_--__- 231 Kaolinite (78.8 percent sodium saturated) in distilled Clay minerals._________________________________ 231 water ___-_-____--_-__-__-------_-_---_--____ 246 Phosphate deflocculents_--_-_______-_-__--______ 231 Kaolinite (78.8 percent sodium saturated) in medium- Waters.--____-----_--_--___-_-__--_-________ 231 hard water...________________________________ 246 Methods_______________________._________ -

A Study on the Genesis of Kaolin Clays of Campo Alegre Basin, Santa Catarina State, Brazil
Anais da Academia Brasileira de Ciências (2007) 79(4): 665-681 (Annals of the Brazilian Academy of Sciences) ISSN 0001-3765 www.scielo.br/aabc Coexistence of halloysite and kaolinite – a study on the genesis of kaolin clays of Campo Alegre Basin, Santa Catarina State, Brazil MARISA T.G. DE OLIVEIRA1, SANDRA M.A. FURTADO2, MILTON L.L. FORMOSO3, RICHARD A. EGGLETON4 and NORBERTO DANI3 1Universidade Federal do Rio Grande do Sul, Instituto de Geociências, Av. Bento Gonçalves, 9500 91509-900 Porto Alegre, RS, Brasil 2Universidade Federal de Santa Catarina, Trindade – 88040-970 Florianópolis, SC, Brasil 3Centro de Estudos em Petrologia e Geoquímica, Instituto de Geociências, Universidade Federal do Rio Grande do Sul Av. Bento Gonçalves, 9500 – 91509-900 Porto Alegre, RS, Brasil 4Department of Earth and Marine Sciences, DEMS Building 047, Australian National University, ACT 0200 Australia Manuscript received on October 13, 2005; accepted for publication on September 10, 2006; contributed by MILTON L.L. FORMOSO* ABSTRACT Kaolin at Campo Alegre Basin, Santa Catarina State, Brazil was formed from alteration of volcanic acid rocks. Halloysite clays dominate the clay fraction of the matrix of the kaolin body, whereas a poorly crystalline kaolinite is abundant in veins. Some primary blocky structures have high amounts of illite, in one mine, but in general, only low contents of illite-smectite, illite, chlorite-vermiculite, vermiculite and quartz were identified in the clay fraction of the samples. Toward the top of the mines, hematite and lepidocrocite appear in horizontal red and ochre colored levels and the amount of kaolinite increases compared to halloysite. The vertical zoning of alteration levels, the changes in mineralogy, the positive correlation between depth and Cation Exchange Capacity of the clays, the preservation of different types of rock textures in the kaolin bodies, the dominant tube morphology of the halloysite clays indicate a supergene genesis for the deposits. -

Clay Minerals in Hydrothermal Systems
minerals Review Clay Minerals in Hydrothermal Systems Paolo Fulignati Dipartimento di Scienze della Terra, University of Pisa, Via S. Maria 53, 56126 Pisa, Italy; [email protected] Received: 24 August 2020; Accepted: 14 October 2020; Published: 16 October 2020 Abstract: The study of active and fossil hydrothermal systems shows clay minerals to be a fundamental tool for the identification and characterization of hydrothermal alteration facies. The occurrence and composition of hydrothermal alteration facies could provide useful information on the physicochemical conditions of the hydrothermal activity affecting a rock volume. In particular, clay minerals (i.e., smectite group, chlorite, illite, kaoline group, pyrophyllite, biotite) are pivotal for extrapolating important parameters that strongly affect the development of water/rock interaction processes such as the temperature and pH of the hydrothermal environment. This work aims to give a general reference scheme concerning the occurrence of clay minerals in hydrothermal alteration paragenesis, their significance, and the information that can be deduced by their presence and chemical composition, with some examples from active and fossil hydrothermal systems around the world. The main mineralogical geothermometers based on chlorite and illite composition are presented, together with the use of hydrogen and oxygen isotope investigation of clay minerals in hydrothermal systems. These techniques provide a useful tool for the reconstruction of the origin and evolution of fluids involved in hydrothermal alteration. Finally, a list of oxygen and hydrogen fractionation factor equations between the main clay minerals and water is also provided. Keywords: clay minerals; hydrothermal alteration; hydrothermal mineralogical paragenesis; geothermometers 1. Introduction A volume of rock, when affected by hydrothermal fluid circulation, typically undergoes a variety of alteration processes. -

Kinetics of Illite Formation
Kinetics of illite formation DENNIS EBERL Department of Geology, University of Illinois at Urbana-Champaign, Urbana, Illinois 61801 JOHN HOWER Department of Geology, Case Western Reserve University, Cleveland, Ohio 44106 ABSTRACT companying increase in positive charge in the interlayer position through the concen- An activation energy of 19.6 ± 3.5 tration of cations such as K+. When the kcal/mole was found for conversion of charge on a layer reaches about 0.7 equi- synthetic beidellite with the composition valents per O10(OH)2 (see Hower and Al2Si3.66Alo.3401o(OH)2K().34 to mixed-layer Mowatt, 1966), water is expelled from the illite/smectite. The size of this activation interlayer and potassium is fixed, convert- energy and the rate constants suggest that ing the expandable smectite structure into (1) the alteration of smectite to illite during that of an illite. diagenesis involves the breaking of chemi- Perry and Hower (1970) suggested that cal bonds in the 2:1 layers; (2) either an the occurrence of this reaction is directly re- equilibrium or a kinetic interpretation for lated to temperature. For example, data the range of mixed layering found in burial from a typical Gulf Coast well is shown in diagenetic sequences is compatible with the Figure 1. Mixed-layer clay from this well is T° C kinetic data; and (3) the formation of illite initially 80 percent expandable (that is, Figure 1. Relationship between expandability from smectite on the ocean floor will not be crystallites contain 80 percent smectite and and temperature for I/S from well "E" of Perry seen, even if the reaction is favored ther- 20 percent illite layers).