LIGAND FORMULA INDEX Order of Elements: C,Il,O,N, Others In
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Download Download
Preparation of Telluric Acid from Tellurium Dioxide by Oxidation with Potassium Permanganate Frank C. Mathers, Charles M. Rice, Howard Broderick, and Robert Forney, Indiana University General Statement Tellurium dioxide, Te0 2 , although periodically similar to sulfur dioxide, cannot be oxidized by nitric acid to the valence of six, i.e., to telluric acid, H 2 Te04.2H 2 0. Among the many stronger oxidizing agents that will produce this oxidation, potassium permanganate in a nitric acid solution is quite satisfactory. This paper gives directions and data for the preparation of telluric acid by this reaction. The making of telluric acid is a desirable laboratory experiment because (1) tellurim dioxide is available in large quantities and is easily obtained, (2) the telluric acid is a stable compound, easily purified, easily crystallized, and non-corrosive, and (3) students are interested in experimenting with the rarer elements. The small soluibility of telluric acid and the high solubility of both manganese and potassium nitrates in nitric acidi gives a sufficient differ- ence in properties for successful purification by crystallization of the telluric acid. Methods of Analyses Tellurium dioxide can be volumetrically 2 titrated in a sulfuric acid solution by an excess of standard potassium permanganate, followed by enough standard oxalic acid to decolorize the excess of permanganate. The excess of oxalic acid must then be titrated by more of the perman- ganate. The telluric acid can be titrated, like any ordinary monobasic acid, 3 (1911). with standard sodium hydroxide using phenolphthalein as an indicator, if an equal volume of glycerine is added. If any nitric acid is present, it must be neutralized first with sodium hydroxide, using methyl orange as indicator. -

Appendix H EPA Hazardous Waste Law
Appendix H EPA Hazardous Waste Law This Appendix is intended to give you background information on hazardous waste laws and how they apply to you. For most U.S. Environmental Protection Agency (EPA) requirements that apply to the University, the Safety Department maintains compliance through internal inspections, record keeping and proper disposal. In Wisconsin, the Department of Natural Resources (DNR) has adopted the EPA regulations, consequently EPA and DNR regulations are nearly identical. EPA defines This Appendix only deals with "hazardous waste" as defined by the EPA. hazardous waste as Legally, EPA defines hazardous waste as certain hazardous chemical waste. This hazardous chemical Appendix does not address other types of regulated laboratory wastes, such as waste; radioactive, infectious, biological, radioactive or sharps. Chapter 8 descibes disposal procedures infectious and biohazardous waste for animals. Chapter 9 describes disposal procedures for sharps and other waste that are regulated by can puncture tissue. Chapter 11 discusses Radiation and the Radiation Safety for other agencies. Radiation Workers provides guidelines for the disposal of radioactive waste. Procedures for medical waste are written by the UW Hospital Safety Officer. The Office of Biological Safety can provide guidance for the disposal of infectious and biological waste. EPA regulations focus on industrial waste streams. As a result, many laboratory chemical wastes are not regulated by EPA as hazardous chemical waste. However, many unregulated chemical wastes do merit special handling and disposal If a waste can be procedures. Thus, Chapter 7 and Appendix A of this Guide recommend disposal defined as: procedures for many unregulated wastes as if they were EPA hazardous waste. -

Phosphorus and Sulfur Cosmochemistry: Implications for the Origins of Life
Phosphorus and Sulfur Cosmochemistry: Implications for the Origins of Life Item Type text; Electronic Dissertation Authors Pasek, Matthew Adam Publisher The University of Arizona. Rights Copyright © is held by the author. Digital access to this material is made possible by the University Libraries, University of Arizona. Further transmission, reproduction or presentation (such as public display or performance) of protected items is prohibited except with permission of the author. Download date 07/10/2021 06:16:37 Link to Item http://hdl.handle.net/10150/194288 PHOSPHORUS AND SULFUR COSMOCHEMISTRY: IMPLICATIONS FOR THE ORIGINS OF LIFE by Matthew Adam Pasek ________________________ A Dissertation Submitted to the Faculty of the DEPARTMENT OF PLANETARY SCIENCE In Partial Fulfillment of the Requirements For the Degree of DOCTOR OF PHILOSOPHY In the Graduate College UNIVERSITY OF ARIZONA 2 0 0 6 2 THE UNIVERSITY OF ARIZONA GRADUATE COLLEGE As members of the Dissertation Committee, we certify that we have read the dissertation prepared by Matthew Adam Pasek entitled Phosphorus and Sulfur Cosmochemistry: Implications for the Origins of Life and recommend that it be accepted as fulfilling the dissertation requirement for the Degree of Doctor of Philosophy _______________________________________________________________________ Date: 04/11/2006 Dante Lauretta _______________________________________________________________________ Date: 04/11/2006 Timothy Swindle _______________________________________________________________________ Date: 04/11/2006 -

Vanadate-Molybdate Reagent Safety Data Sheet According to Federal Register / Vol
Vanadate-Molybdate Reagent Safety Data Sheet according to Federal Register / Vol. 77, No. 58 / Monday, March 26, 2012 / Rules and Regulations Date of issue: 12/20/2013 Revision date: 05/02/2014 Supersedes: 12/20/2013 Version: 1.1 SECTION 1: Identification of the substance/mixture and of the company/undertaking 1.1. Product identifier Product form : Mixture Product name : Vanadate-Molybdate Reagent Product code : LC26600 1.2. Relevant identified uses of the substance or mixture and uses advised against Use of the substance/mixture : For laboratory and manufacturing use only. 1.3. Details of the supplier of the safety data sheet LabChem Inc Jackson's Pointe Commerce Park Building 1000, 1010 Jackson's Pointe Court Zelienople, PA 16063 - USA T 412-826-5230 - F 724-473-0647 [email protected] - www.labchem.com 1.4. Emergency telephone number Emergency number : CHEMTREC: 1-800-424-9300 or 011-703-527-3887 SECTION 2: Hazards identification 2.1. Classification of the substance or mixture GHS-US classification Skin Corr. 1B H314 Eye Dam. 1 H318 2.2. Label elements GHS-US labelling Hazard pictograms (GHS-US) : GHS05 Signal word (GHS-US) : Danger Hazard statements (GHS-US) : H314 - Causes severe skin burns and eye damage Precautionary statements (GHS-US) : P260 - Do not breathe mist, vapours, spray P264 - Wash exposed skin thoroughly after handling P280 - Wear protective gloves, eye protection, protective clothing, face protection P301+P330+P331 - IF SWALLOWED: rinse mouth. Do NOT induce vomiting P303+P361+P353 - IF ON SKIN (or hair): Remove/Take off immediately all contaminated clothing. Rinse skin with water/shower P304+P340 - IF INHALED: remove victim to fresh air and keep at rest in a position comfortable for breathing P305+P351+P338 - If in eyes: Rinse cautiously with water for several minutes. -

Shale Gas and Groundwater Quality
Shale Gas and Groundwater Quality A literature review on fate and effects of added chemicals Alette Langenhoff 1202141-008 © Deltares, 2011 1202141-008-ZWS-0001, 28 December 2011, final Contents 1 Introduction 1 2 The process of fracturing or fracking 5 3 The use of chemicals 7 4 Polyacrylamide 8 4.1 Aerobic degradation of polyacrylamide 8 4.2 Anaerobic degradation 10 4.3 Chemical or physical removal 10 4.4 Conclusion on removal of polyacrylamide 10 5 Glutaraldehyde 12 5.1 Biocide 12 5.2 Biodegradation 12 5.3 Chemical inactivation of glutaraldehyde 13 6 Conclusions 14 7 References 15 Appendices 17 Appendices A Chemicals identified in hydraulic fracturing fluid and flowback/produced water (EPA, 2011). A-1 B Fracturing fluid ingredients and common uses (Europe Unconventional Gas 2011) B-1 C Properties of Polyacrylamide (source: Wikipedia) C-1 D Properties of Glutaraldehyde (source: Wikipedia) D-1 Shale Gas and Groundwater Quality i 1202141-008-ZWS-0001, 28 December 2011, final 1 Introduction Shale gas is a so-called unconventional sources of natural gas, and is one of the most rapidly expanding trends in onshore domestic oil and gas exploration and production today (Fig. 1 and 2). Shale gas is present in hydrocarbon rich shale formations. Shallow gas is commonly defined as gas occurrences in unconsolidated sediments of Tertiary age (often down to depths of 1000 m below surface). The occurrences are positively associated with thick Neogene sediments and are often trapped in anticlinal structures associated with rising salt domes (Muntendam-Bos et al, 2009). Shale has low matrix permeability, so gas production in commercial quantities requires fractures to provide permeability. -

APPENDIX G Acid Dissociation Constants
harxxxxx_App-G.qxd 3/8/10 1:34 PM Page AP11 APPENDIX G Acid Dissociation Constants § ϭ 0.1 M 0 ؍ (Ionic strength ( † ‡ † Name Structure* pKa Ka pKa ϫ Ϫ5 Acetic acid CH3CO2H 4.756 1.75 10 4.56 (ethanoic acid) N ϩ H3 ϫ Ϫ3 Alanine CHCH3 2.344 (CO2H) 4.53 10 2.33 ϫ Ϫ10 9.868 (NH3) 1.36 10 9.71 CO2H ϩ Ϫ5 Aminobenzene NH3 4.601 2.51 ϫ 10 4.64 (aniline) ϪO SNϩ Ϫ4 4-Aminobenzenesulfonic acid 3 H3 3.232 5.86 ϫ 10 3.01 (sulfanilic acid) ϩ NH3 ϫ Ϫ3 2-Aminobenzoic acid 2.08 (CO2H) 8.3 10 2.01 ϫ Ϫ5 (anthranilic acid) 4.96 (NH3) 1.10 10 4.78 CO2H ϩ 2-Aminoethanethiol HSCH2CH2NH3 —— 8.21 (SH) (2-mercaptoethylamine) —— 10.73 (NH3) ϩ ϫ Ϫ10 2-Aminoethanol HOCH2CH2NH3 9.498 3.18 10 9.52 (ethanolamine) O H ϫ Ϫ5 4.70 (NH3) (20°) 2.0 10 4.74 2-Aminophenol Ϫ 9.97 (OH) (20°) 1.05 ϫ 10 10 9.87 ϩ NH3 ϩ ϫ Ϫ10 Ammonia NH4 9.245 5.69 10 9.26 N ϩ H3 N ϩ H2 ϫ Ϫ2 1.823 (CO2H) 1.50 10 2.03 CHCH CH CH NHC ϫ Ϫ9 Arginine 2 2 2 8.991 (NH3) 1.02 10 9.00 NH —— (NH2) —— (12.1) CO2H 2 O Ϫ 2.24 5.8 ϫ 10 3 2.15 Ϫ Arsenic acid HO As OH 6.96 1.10 ϫ 10 7 6.65 Ϫ (hydrogen arsenate) (11.50) 3.2 ϫ 10 12 (11.18) OH ϫ Ϫ10 Arsenious acid As(OH)3 9.29 5.1 10 9.14 (hydrogen arsenite) N ϩ O H3 Asparagine CHCH2CNH2 —— —— 2.16 (CO2H) —— —— 8.73 (NH3) CO2H *Each acid is written in its protonated form. -
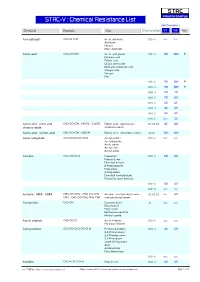
STAC-V : Chemical Resistance List Max Temperature
S TA C Industrial Coatings STAC-V : Chemical Resistance List Max Temperature Chemical Formula Alias Concentration V1 V2 Note Acetaldehyde CH3-CH=O Acetic aldehyde 100 % n.r. n.r. Aldehyde Ethanal Ethyl aldehyde Acetic acid CH3-CO-OH Acetic acid glacial 010 % 90 100 0 Ethanoic acid Ethylic acid Glacial acetic acid Methane carboxylic acid Vinegar acid Vinegar Hac 015 % 90 100 0 025 % 90 100 0 040 % 80 90 050 % 70 80 075 % 60 65 080 % 45 45 085 % 45 45 100 % n.r. 25 Acetic acid : nitric acid : CH3-CO-OH : HNO3 : Cr2O3 Ethylic acid : salpeterzuur : 03:05:03 65 80 chromic oxide chromium oxide Acetic acid : sulfuric acid CH3-CO-OH : H2SO4 Ethylic acid : dihydrogen sulfate 20:10 100 100 Acetic anhydride CH3-CO-O-CO-CH3 Acetyl acetate 100 % n.r. n.r. Acetanhydride Acetic oxide Acetyl ether Acetyl oxide Acetone CH3-CO-CH3 Propanone 005 % 80 80 Propan-2-one Dimethyl ketone β-Ketopropane[ Propanone 2-Propanone Dimethyl formaldehyde Pyroacetic spirit (archaic) 010 % 80 80 100 % n.r. n.r. Acetone : MEK : MiBK CH3-CO-CH3 : CH3-CO-CH2- Acetone : methylethyl ketone : 02:02:02 n.r. 40 CH3 : CH3-CO-CH2-CH2-CH3 methylisobutyl ketone Acetonitrile CH3-CN Cyanomethane all n.r. n.r. Ethanenitrile Ethyl nitrile Methanecarbonitrile Methyl cyanid Acetyl chloride CH3-CO-Cl Acetic chloride 100 % n.r. n.r. Ethanoyl chloride Acetylacetone CH3-CO-CH2-CO-CH3 Pentane-2,4-dione 020 % 40 50 2,4-Pentanedione 2,4-Dioxopentane 2,4-Pentadione acetyl-2-Propanone Acac Acetoacetone Diacetylmethane 100 % n.r. -

Estimation of Hydrolysis Rate Constants of Carboxylic Acid Ester and Phosphate Ester Compounds in Aqueous Systems from Molecular Structure by SPARC
Estimation of Hydrolysis Rate Constants of Carboxylic Acid Ester and Phosphate Ester Compounds in Aqueous Systems from Molecular Structure by SPARC R E S E A R C H A N D D E V E L O P M E N T EPA/600/R-06/105 September 2006 Estimation of Hydrolysis Rate Constants of Carboxylic Acid Ester and Phosphate Ester Compounds in Aqueous Systems from Molecular Structure by SPARC By S. H. Hilal Ecosystems Research Division National Exposure Research Laboratory Athens, Georgia U.S. Environmental Protection Agency Office of Research and Development Washington, DC 20460 NOTICE The information in this document has been funded by the United States Environmental Protection Agency. It has been subjected to the Agency's peer and administrative review, and has been approved for publication. Mention of trade names of commercial products does not constitute endorsement or recommendation for use. ii ABSTRACT SPARC (SPARC Performs Automated Reasoning in Chemistry) chemical reactivity models were extended to calculate hydrolysis rate constants for carboxylic acid ester and phosphate ester compounds in aqueous non- aqueous and systems strictly from molecular structure. The energy differences between the initial state and the transition state for a molecule of interest are factored into internal and external mechanistic perturbation components. The internal perturbations quantify the interactions of the appended perturber (P) with the reaction center (C). These internal perturbations are factored into SPARC’s mechanistic components of electrostatic and resonance effects. External perturbations quantify the solute-solvent interactions (solvation energy) and are factored into H-bonding, field stabilization and steric effects. These models have been tested using 1471 reliable measured base, acid and general base-catalyzed carboxylic acid ester hydrolysis rate constants in water and in mixed solvent systems at different temperatures. -

Hydrolysis and Atmospheric Oxidation Reactions of Perfluorinated Carboxylic Acid Precursors
Hydrolysis and Atmospheric Oxidation Reactions of Perfluorinated Carboxylic Acid Precursors by Derek A. Jackson A thesis submitted in conformity with the requirements for the degree of Doctor of Philosophy Graduate Department of Chemistry University of Toronto © Copyright by Derek A. Jackson 2013 Hydrolysis and Atmospheric Oxidation Reactions of Perfluorinated Carboxylic Acid Precursors Derek Andrew Jackson Doctor of Philosophy Department of Chemistry University of Toronto 2013 Abstract This dissertation explores a number of different environmentally relevant reactions that lead to the production of perfluorocarboxylic acids (PFCAs), a family of environmental pollutants that does not undergo any further degradation pathways. The compound perfluoro-2-methyl-3-pentanone (PFMP) is a new fire fighting fluid developed by 3M that is designed as a Halon replacement. The environment fate of PFMP with regards to direct photolysis, abiotic hydrolysis and hydration was determined using a combination of laboratory experiments and computational modeling. PFMP was found to undergo direct photolysis giving a lifetime of 4-14 days depending on latitude and time of year. Offline samples confirmed PFCA products and a mechanism was proposed. Polyfluorinated amides (PFAMs) are a class of chemicals produced as byproducts of polyfluorinated sulfonamide synthesis via electrochemical fluorination (ECF). Using synthesized standards of four model compounds, PFAMs were detected and quantified in a variety of legacy commercial materials synthesized by ECF. PFAMs were hypothesized to undergo biological hydrolysis reactions, suggesting their importance as historical PFOA precursors. ii The PFAMs were also investigated with regards to their environmental fate upon atmospheric oxidation. Using a smog chamber, the kinetics and degradation mechanisms of N- ethylperfluorobutyramide (EtFBA) were elucidated. -

United States Patent 19 11 4,289,699 Oba Et Al
United States Patent 19 11 4,289,699 Oba et al. 45 Sep. 15, 1981 54 PROCESS FOR THE PRODUCTION OF Primary Examiner-Donald G. Daus N-(HYDROXYPHENYL) MALEIMEDES Assistant Examiner-D. B. Springer 75 Inventors: Masayuki Oba; Motoo Kawamata; Attorney, Agent, or Firm-Fisher, Christen & Sabol Hikotada Tsuboi; Nobuhito Koga, all 57 ABSTRACT of Yokohama, Japan N-(hydroxyphenyl) maleimides of the general formula 73 Assignee: Mitsui Toatsu Chemicals, Incorporated, Tokyo, Japan 21 Appl. No.: 88,825 (22 Filed: Oct. 26, 1979 N CO-m-CH Related U.S. Application Data (HO) 62) Division of Ser. No. 956,971, Nov. 2, 1978, Pat. No. 4,231,934. where R' stands for H, CH3, C2H5, F, Cl, Br or I and in 30 Foreign Application Priority Data is an integer of 1-5 are produced by treating the corre sponding maleamic acid or by treating the ester of said Nov. 2, 1977 (JP) Japan ................................ 52-130905 N-(hydroxyphenyl) maleimide at a temperature of Nov. 4, 1977 (JP Japan ................................ 52-3504 0-150° C. in the presence of at least one dehydrating 51) Int. Cl. .......................................... C07D 207/.452 agent selected for the group consisting of oxides and (52) U.S. Cl. .......................................... 260/326.5 FM oxyacids of sulfur or phosphorus and alkali metal and 58) Field of Search .............................. 260/326.5 FM alkaline earth metal salts of the said oxyacids. The cor responding maleamic acid can be obtained by reacting 56) - References Cited an aminophenol having one or more hydroxyl groups U.S. PATENT DOCUMENTS on its phenyl nucleus with maleic anhydride. -

I Norgan Ic C He Mi Str Y
View Article Online / Journal Homepage / Table of Contents for this issue INORGANIC CHEMISTRY. 443 I n o r g a n ic C h e mi s t r y. Composition of Atmospheres which Extinguish Flame. By FRANKCLOWES ( PTOC. Roy. Soc., 1894, 56, 2-6) .--The experimental flame burning at a platinum jet 1 mm. in diameter, was 0.75 in. in height; it was gradually lowered into a cylinder containing the atmosphere of mixed gases, and these were considered to be in extinctive proportions if the flame was extinguished during its downward passage, or immediately on attaining its lowest position in the cylinder. The gaseous mixture was regarded as containing the minimum quantitr of extinctive gas, when the flame on being lowered into another mixture containing 1 per cent. less of such gas continued to burn in it for a few seconds before being ex- tinguished. Experiments made with flames of hydrogen and Published on 01 January 1895. Downloaded 25/10/2014 07:12:09. alcohol, varying from 0.4 in. to 1.5 in. in height, show that the varying dimensions of the flame are without influence on the proportion of carbonic anhydride in the air necessary to pro- duce extinction. Characteristic differences were observed between the behaviour of wick-fed flames and that of gas-fed flames when they were introduced into an atmosphere which extinguished them, the wick-fed flames gradually diminishing in size until they vanished, whilst the gas-fed flames gradually increased in size, becoming paler and apparently lower in temperature until they suddenly expired. -

Vanadate and Peroxovanadate Complexes of Biomedical Relevance – a Speciation Approach with Focus on Diabetes
Vanadate and Peroxovanadate Complexes of Biomedical Relevance – A speciation approach with focus on diabetes András Gorzsás Department of Chemistry Inorganic Chemistry Umeå University Umeå, Sweden Akademisk avhandling som med tillstånd av rektorsämbetet vid Umeå Universitet för erhållande av Filosofie Doktorsexamen framlägges till offentlig granskning vid Kemiska institutionen, sal KB3A9, KBC–huset, fredagen den 22 april 2005, kl 13.00. Fakultetsopponent: Professor Carlos F. G. C. Geraldes, Department of Biochemistry, Faculty of Science and Technology, University of Coimbra, P. O. Box 3126, P – 3001 – 401 Coimbra, Portugal i TITLE Vanadate and Peroxovanadate Complexes of Biomedical Relevance – A speciation approach with focus on diabetes AUTHOR András Gorzsás ADDRESS Department of Chemistry, Inorganic Chemistry, Umeå University, SE – 901 87 Umeå, Sweden ABSTRACT Diabetes mellitus is one of the most threatening epidemics of modern times with rapidly increasing incidence. Vanadium and peroxovanadium compounds have been shown to exert insulin–like actions and, in contrast to insulin, are orally applicable. However, problems with side–effects and toxicity remain. The exact mechanism(s) by which these compounds act are not yet fully known. Thus, a better understanding of the aqueous chemistry of vanadates and peroxovanadates in the presence of various (bio)ligands is needed. The present thesis summarises six papers dealing mainly with aqueous speciation in different vanadate – and peroxovanadate – ligand systems of biological and medical relevance. Altogether, five ligands have been studied, including important blood constituents (lactate, citrate and phosphate), a potential drug candidate (picolinic acid), and a dipeptide (alanyl serine) to model the interaction of (peroxo)vanadate in the active site of enzymes. Since all five ligands have been studied both with vanadates and peroxovanadates, the number of systems described in the present work is eleven, including the vanadate – citrate – lactate mixed ligand system.