Quality Control on Herbal Medicine and Its Application
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

12.2% 122,000 135M Top 1% 154 4,800
We are IntechOpen, the world’s leading publisher of Open Access books Built by scientists, for scientists 4,800 122,000 135M Open access books available International authors and editors Downloads Our authors are among the 154 TOP 1% 12.2% Countries delivered to most cited scientists Contributors from top 500 universities Selection of our books indexed in the Book Citation Index in Web of Science™ Core Collection (BKCI) Interested in publishing with us? Contact [email protected] Numbers displayed above are based on latest data collected. For more information visit www.intechopen.com Chapter Traditional Chinese Medicine: From Aqueous Extracts to Therapeutic Formulae Jinfan Wang, Astrid Sasse and Helen Sheridan Abstract Traditional Chinese medicine (TCM) is one of the most established systems of medicine in the world. The therapeutic formulae used in TCM are frequently derived from aqueous decoctions of single plants or complex multicomponent formulae. There are aspects of plant cultivation and preparation of decoction pieces that are unique to TCM. These include Daodi cultivation, which is associated with high quality medicinal plant material that is grown in a defined geographical area, and Paozhi processing where the decoction pieces can be treated with excipients and are processed, which may fundamentally change the nature of the chemical metabolites. Therefore, a single plant part, processed in a variety of different ways, can each create a unique medicine. The quality of TCM materials, their safety and therapeutic efficacy are of critical importance. The application of metabolomic and chemometric techniques to these complex and multicomponent medicines is of interest to understand the interrelationships between composition, synergy and therapeutic activity. -

Sustainable Sourcing : Markets for Certified Chinese
SUSTAINABLE SOURCING: MARKETS FOR CERTIFIED CHINESE MEDICINAL AND AROMATIC PLANTS In collaboration with SUSTAINABLE SOURCING: MARKETS FOR CERTIFIED CHINESE MEDICINAL AND AROMATIC PLANTS SUSTAINABLE SOURCING: MARKETS FOR CERTIFIED CHINESE MEDICINAL AND AROMATIC PLANTS Abstract for trade information services ID=43163 2016 SITC-292.4 SUS International Trade Centre (ITC) Sustainable Sourcing: Markets for Certified Chinese Medicinal and Aromatic Plants. Geneva: ITC, 2016. xvi, 141 pages (Technical paper) Doc. No. SC-2016-5.E This study on the market potential of sustainably wild-collected botanical ingredients originating from the People’s Republic of China with fair and organic certifications provides an overview of current export trade in both wild-collected and cultivated botanical, algal and fungal ingredients from China, market segments such as the fair trade and organic sectors, and the market trends for certified ingredients. It also investigates which international standards would be the most appropriate and applicable to the special case of China in consideration of its biodiversity conservation efforts in traditional wild collection communities and regions, and includes bibliographical references (pp. 139–140). Descriptors: Medicinal Plants, Spices, Certification, Organic Products, Fair Trade, China, Market Research English For further information on this technical paper, contact Mr. Alexander Kasterine ([email protected]) The International Trade Centre (ITC) is the joint agency of the World Trade Organization and the United Nations. ITC, Palais des Nations, 1211 Geneva 10, Switzerland (www.intracen.org) Suggested citation: International Trade Centre (2016). Sustainable Sourcing: Markets for Certified Chinese Medicinal and Aromatic Plants, International Trade Centre, Geneva, Switzerland. This publication has been produced with the financial assistance of the European Union. -

Triterpenoid Saponins from the Roots of Cyathula Officinalis and Their Inhibitory Effects on Nitric Oxide Production
Chinese Journal of Natural Chinese Journal of Natural Medicines 2017, 15(6): 04630466 Medicines doi: 10.3724/SP.J.1009.2017.00463 Triterpenoid saponins from the roots of Cyathula officinalis and their inhibitory effects on nitric oxide production JIANG Yun-Tao1, 2, YAN Wen-Jing1, 2, QI Chu-Lu1, 2, HOU Ji-Qin1, 2, ZHONG Yan-Ying1, 2, LI Hui-Jun1, WANG Hao1, 2 *, LI Ping1 1 State Key Laboratory of Natural Medicines, China Pharmaceutical University, Nanjing 210009, China; 2 Department of Natural Medicinal Chemistry, China Pharmaceutical University, Nanjing 210009, China Available online 20 Jun., 2017 [ABSTRACT] The present study was designed to investigate the chemical constituents of the roots of Cyathula officinalis. Compounds were isolated by silica gel, Sephadex LH-20, ODS column chromatography, and preparative HPLC. Their structures were determined on the basis of 1D and 2D NMR techniques, mass spectrometry, and chemical methods. One new oleanane-type triterpenoid saponin, 28-O-[α-L-rhamno- pyranosyl-(1→3)-β-D-glucuronopyranosyl-(1→3)-β-D-glucopyranosyl] hederagenin (1), was isolated from the roots of Cyathula offici- nalis. The anti-inflammatory activities of the isolates were evaluated for their inhibitory effects against LPS-induced nitric oxide (NO) production in RAW 264.7 macrophages cells. Compounds 2, 4, and 6 exhibited moderate anti-inflammatory activities. [KEY WORDS] Cyathula officinalis; Amaranthaceae; Triterpenoid saponins; Nitric oxide inhibition [CLC Number] R284 [Document code] A [Article ID] 2095-6975(2017)06-0463-04 hibitory effects on NO production in LPS-stimulated RAW 264.7 Introduction macrophages cells. Cyathula officinalis Kuan belongs to Amaranthaceae Results and Discussion family and grows in the southwest of China. -

Amaranthaceae.Pdf
Flora of China 9: 415-429. 2003. AMARANTHACEAE 苋科 xian ke Bao Bojian (包伯坚)1; Steven E. Clemants2, Thomas Borsch3 Herbs, clambering subshrubs, shrubs, or lianas. Leaves alternate or opposite, entire, exstipulate. Flowers small, bisexual or unisexual, or sterile and reduced, subtended by 1 membranous bract and 2 bracteoles, solitary or aggregated in cymes. Inflorescences elongated or condensed spikes (heads), racemes, or thyrsoid structures of varying complexity. Bracteoles membranous or scarious. Tepals 3–5, membranous, scarious or subleathery, 1-, 3-, 5-, or 7(–23)-veined. Stamens as many as tepals and opposite these, rarely fewer than tepals; filaments free, united into a cup at base or ± entirely into a tube, filament lobes present or absent, pseudostaminodes present or absent; anthers (1- or)2-loculed, dorsifixed, introrsely dehiscent. Ovary superior, 1-loculed; ovules 1 to many; style persistent, short and indistinct or long and slender; stigma capitate, penicillate, 2-lobed or forming 2 filiform branches. Fruit a dry utricle or a fleshy capsule, indehiscent, irregularly bursting, or circumscissile. Seeds lenticular, reniform, subglobose, or shortly cylindric, smooth or verruculose. About 70 genera and 900 species: worldwide; 15 genera (one introduced) and 44 species (three endemic, 14 introduced) in China. Morphology of the androecium, perianth (tepals), and the inflorescence has traditionally been used to circumscribe genera and tribes. Pseudostaminodia are interstaminal appendages with variously shaped apices. Filament appendages are the lateral appendages of filaments (one on each side). The basic structure of the inflorescence is the cyme (branchlets arising from the bracteole axils, the bracteoles serving as bracts for upper flowers), which can be reduced to one flower with two bracteoles and a bract. -

Compilation of the Literature Reports for the Screening of Vascular Plants, Algae, Fungi and Non- Arthropod Invertebrates for the Presence of Ecdysteroids
COMPILATION OF THE LITERATURE REPORTS FOR THE SCREENING OF VASCULAR PLANTS, ALGAE, FUNGI AND NON- ARTHROPOD INVERTEBRATES FOR THE PRESENCE OF ECDYSTEROIDS Compiled by Laurie Dinan and René Lafont Biophytis, Sorbonne Université, Campus P&M Curie, 4 Place Jussieu, F-75252 Paris Cedex 05, France. Version 6: 24/10/2019 Important notice: This database has been designed as a tool to help the scientific community in research on ecdysteroids. The authors wish it to be an evolving system and would encourage other researchers to submit new data, additional publications, proposals for modifications or comments to the authors for inclusion. All new material will be referenced to its contributor. Reproduction of the material in this database in its entirety is not permitted. Reproduction of parts of the database is only permitted under the following conditions: • reproduction is for personal use, for teaching and research, but not for distribution to others • reproduction is not for commercial use • the origin of the material is indicated in the reproduction • we should be notified in advance to allow us to document that the reproduction is being made Where data are reproduced in published texts, they should be acknowledged by the reference: Lafont R., Harmatha J., Marion-Poll F., Dinan L., Wilson I.D.: The Ecdysone Handbook, 3rd edition, on-line, http://ecdybase.org Illustrations may not under any circumstances be used in published texts, commercial or otherwise, without previous written permission of the author(s). Please notify Laurie Dinan ([email protected]) of any errors or additional literature sources. © 2007: Laurence Dinan and René Lafont CONTENTS 1. -

Development of Successive Cambia and Structure of the Secondary Xylem in Some Members of the Family Amaranthaceae
Plant Science Today (2019) 6(1): 31-39 31 https://doi.org/10.14719/pst.2019.6.1.423 ISSN: 2348-1900 Plant Science Today http://www.plantsciencetoday.online Research Article Development of successive cambia and structure of the secondary xylem in some members of the family Amaranthaceae Ravindra A. Shelke1, Dhara G. Ramoliya2, Amit D. Gondaliya2 and Kishore S. Rajput2* 1Kisan Arts, Commerce and Science College, Parola 425 111, Maharashtra, India 2Department of Botany, Faculty of Science, The Maharaja Sayajirao University of Baroda; Vadodara - 390 002, Gujarat, India Article history Abstract Received: 09 October 2018 Young stems of Aerva javanica (Burm.f.) Juss. ex Schult., A. lanata (L.) Juss. ex Schult, Accepted: 02 January 2019 A. monsonia Mart., A. sanguinolenta (L.) Blume, Alternanthera bettzickiana (Regel) G. Published: 16 January 2019 Nicholson, A. philoxeroides (Mart.) Griseb., Gomphrena celosioides Mart., G. globosa L. and Telanthera ficoidea (L.) Moq., showed the renewal of small sectors of cambium by replacing with new segments. Therefore, the secondary phloem formed by earlier cambial segments form isolated islands of phloem enclosed within conjunctive tissues became embedded in the secondary xylem. As the stem grows older, complete ring of cambium is renewed; sometimes an anastomosing network of successive cambia may be seen due to the renewal of larger segments of the cambium. Renewal of the Editor cambium takes place by repeated periclinal division in the parenchyma cells Dr Sheikh Muhammad Masum positioned outside to the phloem formed by the previous cambium. Functionally the Sher-e-Bangla Agricultural cambium is bidirectional and exclusively composed of fusiform cambial cells. -

Anonymous Classics: a List of Uniform Titles for Chinese Works
INTERNATIONAL FEDERATION OF LIBRARY ASSOCIATIONS AND INSTITUTIONS Cataloguing Section Anonymous classics a list of uniform titles for Chinese works 2011 INTRODUCTION As a reply to the International Conference on Cataloguing Principles, held at UNESCO, Paris, 1961, a first list of uniform titles for Anonymous classics was published by IFLA in 1964 1 with the assistance of UNESCO. This list, which represented a first attempt at the standardization of headings for titles at an international level, was regarded as temporary by its compiler Roger Pierrot. The project was to draw a list of the works from countries which had attended the International Conference: on the basis of the answers provided by 15 European and Asian countries, works of literature in 34 languages were listed in this publication. No later edition was issued for this working tool. In 1978, it was partly replaced by an edition limited to European literatures, revised and augmented by the addition of 8 literatures missing in the 1964 edition. It was planned by the International Office for UBC and coordinated by Rosemary C. Hewett 2. In the early 90s, these two documents were out of print and a mere reprint was not conceivable because of their incompleteness. During the 60th IFLA Conference in Havana in 1994, the Section on Cataloguing included in its action plan a new edition of Anonymous Classics, divided in several phases. The first phase was a revision of existing lists of European literatures, complemented by the addition of literatures missing in the 1978 edition. Published on the IFLA website in 2004, the new edition of Anonymous classics : a list of uniform headings for European literatures includes literatures in 28 languages of the European continent and is completed by definitions of the works and a bibliography for each literature. -

Development and Trade of Medicinal and Aromatic Plants (Maps): Learnings from Comparative Analysis of China and India
Development and Trade of Medicinal and Aromatic Plants (MAPs): Learnings from Comparative Analysis of China and India Study Report Submitted to Rajiv Gandhi Institute of Contemporary Studies (RGICS) Rajiv Gandhi Foundation New Delhi Submitted by Institute of Livelihood Research and Training Regional Office: E-8/23 Vasant Kunj, Arera Colony, Bhopal – 462 039 Website: www.ilrtindia.org Table of Contents List of Tables ................................................................................................................................................. v List of Boxes .................................................................................................................................................. v Abbreviations ............................................................................................................................................... vi Acknowledgement ....................................................................................................................................... ix Executive Summary ....................................................................................................................................... x Chapter 1 ....................................................................................................................................................... 1 Introduction .................................................................................................................................................. 1 1.1. Background ....................................................................................................................................... -

Volume 1 (2016)
VOLUME 1, SPRING 2016 Journal of Asian Humanities at Kyushu University ENVISIONING HISTORY Journal of Asian Humanities at Kyushu University VOLUME 1, SPRING 2016 THEME: ENVISIONING HISTORY CYNTHEA J. BOGEL, VOLUME EDITOR The Journal of Asian Humanities at Kyushu University (JAH-Q) is a peer-reviewed journal published by Kyushu University, School of Letters, Graduate School of Humanities, Faculty of Humanities 九州大学文学部 大学院人文科学府 大学院人文科学研究院. Copyright © 2016 Kyushu University Journal of Asian Humanities at Kyushu University Editorial Board Editor Design Cynthea J. Bogel (Kyushu University) Thomas Eykemans Managing Editor Special thanks for Volume 1, Spring 2016 Tomoyuki Kubo (Kyushu University) Lindsey DeWitt (Kyushu University) John Stevenson (editing) Volume Editor, Envisioning History Cynthea J. Bogel For information about submissions contact: Advisory Members Karl Friday (Saitama University) Shomu Gakari (General Affairs Division), Seinosuke Ide (Kyushu University) Faculty of Humanities, Kyushu University Fabio Rambelli (University of California, Santa Barbara) Address: 6-19-1 Hakozaki, Higashi-ku, Yasutoshi Sakaue (Kyushu University) Fukuoka 812-8581 Japan Takeshi Shizunaga (Kyushu University) Telephone: +81-92-642-2352 Fax: +81-92-642-2349 Melanie Trede (Heidelberg University) E-mail: [email protected] Ellen Van Goethem (Kyushu University) Catherine Vance Yeh (Boston University) Contents VOLUME 1, SPRING 2016 YASUTOSHI SAKAUE Prefatory Note . iv CYNTHEA J. BOGEL Editorial Foreword: Welcome to JAH-Q . v Articles ELLEN VAN GOETHEM WILLIAM MATSUDA Of Trees and Beasts: Site Selection The Birth of Kūkai as a Literary Figure: in Premodern East Asia . 1 A Translation and Analysis of Shinzei’s Preface to the Henjō Hokki Shōryōshū . 29 FLORIAN C. REITER Considerations of Thunder Magic LISA KOCHINSKI Rituals and Thunder Divinities . -

SCIENCE CHINA Induction of Seed Germination in Orobanche Spp. By
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Springer - Publisher Connector SCIENCE CHINA Life Sciences • RESEARCH PAPER • March 2012 Vol.55 No.3: 250–260 doi: 10.1007/s11427-012-4302-2 Induction of seed germination in Orobanche spp. by extracts of traditional Chinese medicinal herbs 1,2* 1 1 3 1 1 MA YongQing , ZHANG Wei , DONG ShuQi , REN XiangXiang , AN Yu & LANG Ming 1College of Resources and Environment, Northwest A & F University, Yangling 712100, China; 2State Key Laboratory of Soil Erosion and Dryland Farming on the Loess Plateau, Institute of Soil and Water Conservation, Northwest A & F University, Yangling 712100, China; 3College of Forestry, Northwest A & F University, Yangling 712100, China Received September 23, 2011; accepted March 5, 2012 The co-evolution of Orobanche spp. and their hosts within the same environment has resulted in a high degree of adaptation and effective parasitism whereby the host releases parasite germination stimulants, which are likely to be unstable in the soil. Our objective was to investigate whether extracts from non-host plants, specifically, Chinese medicinal plants, could stimulate germination of Orobanche spp. Samples of 606 Chinese medicinal herb species were extracted with deionized water and methanol. The extracts were used to induce germination of three Orobanche species; Orobanche minor, Orobanche cumana, and Orobanche aegyptiaca. O. minor exhibited a wide range of germination responses to the various herbal extracts. O. cuma- na and O. aegyptiaca exhibited an intermediate germination response to the herbal extracts. O. minor, which has a narrow host spectrum, showed higher germination rates in response to different herbal extracts compared with those of O. -

Adaptogenic and Anabolic Botanicals to Promote Allostasis
Adaptogenic and Anabolic Botanicals to Promote Allostasis Co-authored by Donald R. Yance Jr., RH (AHG), CN and Suzanne E. Sky, L.Ac., MTOM Table of Contents Part I: Context and Systems ................................................................................................................ 3 Restorative Herbal Medicine: Historical Context .............................................................................3 Ayurvedic and Chinese Restorative Medicine .............................................................................3 Adaptogenic Botanicals and Allostasis ........................................................................................ 3 Homeostasis, Allostasis, and Adaptation .......................................................................................... 4 Selye’s Biological Stress Response ............................................................................................... 4 Allostasis .................................................................................................................................... 5 Metabolic Model of Homeostasis ............................................................................................... 7 Stress Response Systems ...................................................................................................................8 Neuroendocrine Stress Response ................................................................................................ 8 Metabolic Stress Response ..........................................................................................................8 -
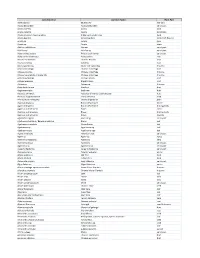
Testing Services List V2020
Latin Binomial Common Name Plant Part Abies sibirica Siberian Fir leaf (oil) Acacia Berlandieri Acacia Berlandieri aerial part Acacia catechu Acacia bark Acacia catechu Acacia gum/resin Acacia nilotica / Acacia arabica Indian gum arabic tree bark Acacia Rigidula Acacia Rigidula herb (leaf, flower) Acacia sp. Acacia gum Acacia sp. Acacia stem Achillea millefolium Yarrow aerial part Achillea sp. Achillea sp. aerial part Achyranthes aspera Prickly chaff flower aerial part Achyranthes bidentata Achyranthes root Aconite carmichaeli Chinese Aconite root Acorus calamus Calamus root Acorus gramineus Grass-leaf sweetflag rhizome Actaea cimicifuga Chinese cimicifuga root Actaea dahurica Chinese cimicifuga rhizome Actaea heracleifolia / Sheng Ma Chinese cimicifuga rhizome Actaea podocarpa Yellow Cohosh root Actaea racemosa Black Cohosh root Actaea sp. Actaea sp. rhizome Actinidia deliciosa Kiwifruit fruit Aegle marmelos Bael tree fruit Aesculus chinensis Aesculus chinensis [Sapindaceae] fruit Aesculus hippocastanum Horse chestnut seed Aframomum melegueta Grains of paradise grain Agaricus bisporus Button Mushroom entire Agaricus bisporus Button Mushroom fruiting body Agaricus subrufescens Blazei entire Agaricus subrufescens Blazei fruiting body Agaricus subrufescens Blazei mycelia Agastache rugosa Huo Xiang aerial part Agathosma betulina / Barosma betulina Buchu leaf Agathosma crenulata Ovate Buchu leaf Agathosma sp. Agathosma sp. leaf Agathosma spp. Agathosma spp. leaf Agave americana American aloe aerial part Agave sp. Agave sp. syrup Agrimonia