Triterpenoid Saponins from the Roots of Cyathula Officinalis and Their Inhibitory Effects on Nitric Oxide Production
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Sustainable Sourcing : Markets for Certified Chinese
SUSTAINABLE SOURCING: MARKETS FOR CERTIFIED CHINESE MEDICINAL AND AROMATIC PLANTS In collaboration with SUSTAINABLE SOURCING: MARKETS FOR CERTIFIED CHINESE MEDICINAL AND AROMATIC PLANTS SUSTAINABLE SOURCING: MARKETS FOR CERTIFIED CHINESE MEDICINAL AND AROMATIC PLANTS Abstract for trade information services ID=43163 2016 SITC-292.4 SUS International Trade Centre (ITC) Sustainable Sourcing: Markets for Certified Chinese Medicinal and Aromatic Plants. Geneva: ITC, 2016. xvi, 141 pages (Technical paper) Doc. No. SC-2016-5.E This study on the market potential of sustainably wild-collected botanical ingredients originating from the People’s Republic of China with fair and organic certifications provides an overview of current export trade in both wild-collected and cultivated botanical, algal and fungal ingredients from China, market segments such as the fair trade and organic sectors, and the market trends for certified ingredients. It also investigates which international standards would be the most appropriate and applicable to the special case of China in consideration of its biodiversity conservation efforts in traditional wild collection communities and regions, and includes bibliographical references (pp. 139–140). Descriptors: Medicinal Plants, Spices, Certification, Organic Products, Fair Trade, China, Market Research English For further information on this technical paper, contact Mr. Alexander Kasterine ([email protected]) The International Trade Centre (ITC) is the joint agency of the World Trade Organization and the United Nations. ITC, Palais des Nations, 1211 Geneva 10, Switzerland (www.intracen.org) Suggested citation: International Trade Centre (2016). Sustainable Sourcing: Markets for Certified Chinese Medicinal and Aromatic Plants, International Trade Centre, Geneva, Switzerland. This publication has been produced with the financial assistance of the European Union. -

Amaranthaceae.Pdf
Flora of China 9: 415-429. 2003. AMARANTHACEAE 苋科 xian ke Bao Bojian (包伯坚)1; Steven E. Clemants2, Thomas Borsch3 Herbs, clambering subshrubs, shrubs, or lianas. Leaves alternate or opposite, entire, exstipulate. Flowers small, bisexual or unisexual, or sterile and reduced, subtended by 1 membranous bract and 2 bracteoles, solitary or aggregated in cymes. Inflorescences elongated or condensed spikes (heads), racemes, or thyrsoid structures of varying complexity. Bracteoles membranous or scarious. Tepals 3–5, membranous, scarious or subleathery, 1-, 3-, 5-, or 7(–23)-veined. Stamens as many as tepals and opposite these, rarely fewer than tepals; filaments free, united into a cup at base or ± entirely into a tube, filament lobes present or absent, pseudostaminodes present or absent; anthers (1- or)2-loculed, dorsifixed, introrsely dehiscent. Ovary superior, 1-loculed; ovules 1 to many; style persistent, short and indistinct or long and slender; stigma capitate, penicillate, 2-lobed or forming 2 filiform branches. Fruit a dry utricle or a fleshy capsule, indehiscent, irregularly bursting, or circumscissile. Seeds lenticular, reniform, subglobose, or shortly cylindric, smooth or verruculose. About 70 genera and 900 species: worldwide; 15 genera (one introduced) and 44 species (three endemic, 14 introduced) in China. Morphology of the androecium, perianth (tepals), and the inflorescence has traditionally been used to circumscribe genera and tribes. Pseudostaminodia are interstaminal appendages with variously shaped apices. Filament appendages are the lateral appendages of filaments (one on each side). The basic structure of the inflorescence is the cyme (branchlets arising from the bracteole axils, the bracteoles serving as bracts for upper flowers), which can be reduced to one flower with two bracteoles and a bract. -

Compilation of the Literature Reports for the Screening of Vascular Plants, Algae, Fungi and Non- Arthropod Invertebrates for the Presence of Ecdysteroids
COMPILATION OF THE LITERATURE REPORTS FOR THE SCREENING OF VASCULAR PLANTS, ALGAE, FUNGI AND NON- ARTHROPOD INVERTEBRATES FOR THE PRESENCE OF ECDYSTEROIDS Compiled by Laurie Dinan and René Lafont Biophytis, Sorbonne Université, Campus P&M Curie, 4 Place Jussieu, F-75252 Paris Cedex 05, France. Version 6: 24/10/2019 Important notice: This database has been designed as a tool to help the scientific community in research on ecdysteroids. The authors wish it to be an evolving system and would encourage other researchers to submit new data, additional publications, proposals for modifications or comments to the authors for inclusion. All new material will be referenced to its contributor. Reproduction of the material in this database in its entirety is not permitted. Reproduction of parts of the database is only permitted under the following conditions: • reproduction is for personal use, for teaching and research, but not for distribution to others • reproduction is not for commercial use • the origin of the material is indicated in the reproduction • we should be notified in advance to allow us to document that the reproduction is being made Where data are reproduced in published texts, they should be acknowledged by the reference: Lafont R., Harmatha J., Marion-Poll F., Dinan L., Wilson I.D.: The Ecdysone Handbook, 3rd edition, on-line, http://ecdybase.org Illustrations may not under any circumstances be used in published texts, commercial or otherwise, without previous written permission of the author(s). Please notify Laurie Dinan ([email protected]) of any errors or additional literature sources. © 2007: Laurence Dinan and René Lafont CONTENTS 1. -

Development of Successive Cambia and Structure of the Secondary Xylem in Some Members of the Family Amaranthaceae
Plant Science Today (2019) 6(1): 31-39 31 https://doi.org/10.14719/pst.2019.6.1.423 ISSN: 2348-1900 Plant Science Today http://www.plantsciencetoday.online Research Article Development of successive cambia and structure of the secondary xylem in some members of the family Amaranthaceae Ravindra A. Shelke1, Dhara G. Ramoliya2, Amit D. Gondaliya2 and Kishore S. Rajput2* 1Kisan Arts, Commerce and Science College, Parola 425 111, Maharashtra, India 2Department of Botany, Faculty of Science, The Maharaja Sayajirao University of Baroda; Vadodara - 390 002, Gujarat, India Article history Abstract Received: 09 October 2018 Young stems of Aerva javanica (Burm.f.) Juss. ex Schult., A. lanata (L.) Juss. ex Schult, Accepted: 02 January 2019 A. monsonia Mart., A. sanguinolenta (L.) Blume, Alternanthera bettzickiana (Regel) G. Published: 16 January 2019 Nicholson, A. philoxeroides (Mart.) Griseb., Gomphrena celosioides Mart., G. globosa L. and Telanthera ficoidea (L.) Moq., showed the renewal of small sectors of cambium by replacing with new segments. Therefore, the secondary phloem formed by earlier cambial segments form isolated islands of phloem enclosed within conjunctive tissues became embedded in the secondary xylem. As the stem grows older, complete ring of cambium is renewed; sometimes an anastomosing network of successive cambia may be seen due to the renewal of larger segments of the cambium. Renewal of the Editor cambium takes place by repeated periclinal division in the parenchyma cells Dr Sheikh Muhammad Masum positioned outside to the phloem formed by the previous cambium. Functionally the Sher-e-Bangla Agricultural cambium is bidirectional and exclusively composed of fusiform cambial cells. -

SCIENCE CHINA Induction of Seed Germination in Orobanche Spp. By
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Springer - Publisher Connector SCIENCE CHINA Life Sciences • RESEARCH PAPER • March 2012 Vol.55 No.3: 250–260 doi: 10.1007/s11427-012-4302-2 Induction of seed germination in Orobanche spp. by extracts of traditional Chinese medicinal herbs 1,2* 1 1 3 1 1 MA YongQing , ZHANG Wei , DONG ShuQi , REN XiangXiang , AN Yu & LANG Ming 1College of Resources and Environment, Northwest A & F University, Yangling 712100, China; 2State Key Laboratory of Soil Erosion and Dryland Farming on the Loess Plateau, Institute of Soil and Water Conservation, Northwest A & F University, Yangling 712100, China; 3College of Forestry, Northwest A & F University, Yangling 712100, China Received September 23, 2011; accepted March 5, 2012 The co-evolution of Orobanche spp. and their hosts within the same environment has resulted in a high degree of adaptation and effective parasitism whereby the host releases parasite germination stimulants, which are likely to be unstable in the soil. Our objective was to investigate whether extracts from non-host plants, specifically, Chinese medicinal plants, could stimulate germination of Orobanche spp. Samples of 606 Chinese medicinal herb species were extracted with deionized water and methanol. The extracts were used to induce germination of three Orobanche species; Orobanche minor, Orobanche cumana, and Orobanche aegyptiaca. O. minor exhibited a wide range of germination responses to the various herbal extracts. O. cuma- na and O. aegyptiaca exhibited an intermediate germination response to the herbal extracts. O. minor, which has a narrow host spectrum, showed higher germination rates in response to different herbal extracts compared with those of O. -
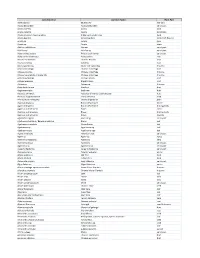
Testing Services List V2020
Latin Binomial Common Name Plant Part Abies sibirica Siberian Fir leaf (oil) Acacia Berlandieri Acacia Berlandieri aerial part Acacia catechu Acacia bark Acacia catechu Acacia gum/resin Acacia nilotica / Acacia arabica Indian gum arabic tree bark Acacia Rigidula Acacia Rigidula herb (leaf, flower) Acacia sp. Acacia gum Acacia sp. Acacia stem Achillea millefolium Yarrow aerial part Achillea sp. Achillea sp. aerial part Achyranthes aspera Prickly chaff flower aerial part Achyranthes bidentata Achyranthes root Aconite carmichaeli Chinese Aconite root Acorus calamus Calamus root Acorus gramineus Grass-leaf sweetflag rhizome Actaea cimicifuga Chinese cimicifuga root Actaea dahurica Chinese cimicifuga rhizome Actaea heracleifolia / Sheng Ma Chinese cimicifuga rhizome Actaea podocarpa Yellow Cohosh root Actaea racemosa Black Cohosh root Actaea sp. Actaea sp. rhizome Actinidia deliciosa Kiwifruit fruit Aegle marmelos Bael tree fruit Aesculus chinensis Aesculus chinensis [Sapindaceae] fruit Aesculus hippocastanum Horse chestnut seed Aframomum melegueta Grains of paradise grain Agaricus bisporus Button Mushroom entire Agaricus bisporus Button Mushroom fruiting body Agaricus subrufescens Blazei entire Agaricus subrufescens Blazei fruiting body Agaricus subrufescens Blazei mycelia Agastache rugosa Huo Xiang aerial part Agathosma betulina / Barosma betulina Buchu leaf Agathosma crenulata Ovate Buchu leaf Agathosma sp. Agathosma sp. leaf Agathosma spp. Agathosma spp. leaf Agave americana American aloe aerial part Agave sp. Agave sp. syrup Agrimonia -

Quality Control on Herbal Medicine and Its Application
Evidence-Based Complementary and Alternative Medicine Quality Control on Herbal Medicine and its Application Lead Guest Editor: Gallant K. L. Chan Guest Editors: Rentian Wu, Vicky P. Chen, Kevin Y. Zhu, and Ying Q. Du Quality Control on Herbal Medicine and its Application Evidence-Based Complementary and Alternative Medicine Quality Control on Herbal Medicine and its Application LeadGuestEditor:GallantK.L.Chan Guest Editors: Rentian Wu, Vicky P. Chen, Kevin Y. Zhu, andYingQ.Du Copyright © 2018 Hindawi. All rights reserved. This is a special issue published in “Evidence-Based Complementary and Alternative Medicine.” All articles are open access articles distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Editorial Board Mona Abdel-Tawab, Germany Kieran Cooley, Canada Cory S. Harris, Canada Rosaria Acquaviva, Italy Edwin L. Cooper, USA Thierry Hennebelle, France GabrielA.Agbor,Cameroon Maria T. Cruz, Portugal Markus Horneber, Germany U. Paulino Albuquerque, Brazil RobertoK.N.Cuman,Brazil Ching-Liang Hsieh, Taiwan Samir Lutf Aleryani, USA Vincenzo De Feo, Italy BennyT.K.Huat,Singapore M. S. Ali-Shtayeh, Palestine Rocío De la Puerta, Spain Helmut Hugel, Australia Gianni Allais, Italy Laura De Martino, Italy Ciara Hughes, Ireland Terje Alraek, Norway AntonioC.P.deOliveira,Brazil Attila Hunyadi, Hungary Isabel Andújar, Spain Arthur De Sá Ferreira, Brazil H. Stephen Injeyan, Canada Letizia Angiolella, Italy Nunziatina De Tommasi, Italy Chie Ishikawa, Japan Makoto Arai, Japan Alexandra Deters, Germany Angelo A. Izzo, Italy Hyunsu Bae, Republic of Korea Farzad Deyhim, USA G. K. Jayaprakasha, USA Giacinto Bagetta, Italy Claudia Di Giacomo, Italy Takahide Kagawa, Japan Onesmo B. -

Evaluation of the Efficacy of a Chinese Herbal Medicine in the Treatment of Patients with Osteoarthritis of the Knee
Evaluation of the efficacy of a Chinese herbal medicine in the treatment of patients with osteoarthritis of the knee Degree title: Doctorate of Philosophy School: School of Biomedical and Health Sciences University: Victoria University, Australia Candidate: Bin Hua Supervisors: Dr. Kylie O’Brien and Dr. Alan Hayes Submission Date: 25 March 2011 Classification Date: 30 January 2012 Abstract Background Osteoarthritis (OA) is common but has no outright cure. Current therapeutic drugs mainly treat OA symptoms and often cause undesirable side effects. Chinese medicine (CM) is a popular alternative therapy for OA, however the majority of CM efficacy studies have been methodologically inadequate. CM has traditionally treated OA under the clinical descriptor of ‘Bi Syndrome’ (painful obstruction syndrome) which includes a range of musculoskeletal disorders. An emerging theory treats OA as a combination of two types of CM Syndromes: Bi Syndrome and Wei Syndrome (atrophy syndrome). There is a lack of objective evidence with respect to possible CM Syndromes of OA, the reliability of CM diagnosis and efficacy of CM treatment guided by this emerging theory. Objectives To investigate the efficacy and safety of a Chinese herbal medicine (CHM) formula developed on the basis of this emerging theory in the treatment of symptoms of knee OA; to investigate the reliability of CM diagnosis in OA patients. Methods A double blind, randomised, placebo controlled clinical trial was conducted in eligible Australian OA patients. Participants were randomised to receive either CHM or placebo over 12 weeks, with a one month follow-up. The Western Ontario and McMaster Universities Arthritis Index (WOMAC) was the primary outcome variable. -

Whole Extracts of Radix Achyranthis Bidentatae and Radix Cyathulae Promote Angiogenesis in Human Umbilical Vein Endothelial Cells in Vitro and in Zebrafish in Vivo
1032 EXPERIMENTAL AND THERAPEUTIC MEDICINE 13: 1032-1038, 2017 Whole extracts of Radix Achyranthis Bidentatae and Radix Cyathulae promote angiogenesis in human umbilical vein endothelial cells in vitro and in zebrafish in vivo XUELIN ZHOU1-3, WING-SUM SIU1-3, CHENG ZHANG1,2, CHEUK-LUN LIU1,2, LING CHENG1,2, HIN-FAI KWOK1,2, CHAK-HEI FUNG1-3, JACQUELINE CHOR-WING TAM1,2, CHING-PO LAU1,2, CLARA BIK-SAN LAU1-3, PING-CHUNG LEUNG1-4, LEUNG-KIM HUNG4 and CHUN-HAY KO1-3 1Institute of Chinese Medicine; 2State Key Laboratory of Phytochemistry and Plant Resources in West China, Shatin, The Chinese University of Hong Kong, Shatin, New Territories; 3Shenzhen Research Institute, The Chinese University of Hong Kong, Shenzhen, Guangdong; 4Department of Orthopaedics and Traumatology, The Chinese University of Hong Kong, Shatin, New Territories, Hong Kong SAR, P.R. China Received August 20, 2015; Accepted November 4, 2016 DOI: 10.3892/etm.2017.4053 Abstract. Although Radix Achyranthis Bidentatae (RAB) HUVECs in vitro and in zebrafish in vivo via increasing cell and Radix Cyathulae (RC) are from two different medicinal migration. plants, they are both used as ‘Niu-Xi’, a widely used traditional Chinese medicine that is believed to stimulate menstruation Introduction and affect bone injury. Angiogenesis is actively involved in treating these illnesses. The aim of the present study was to Angiogenesis is a critical physiological process responsible investigate whether the whole extracts of RAB and RC possess for forming new blood vessels from existing blood vessels (1). pro-angiogenic effects. In order to examine this idea whole Endothelial cells (ECs) evidently participate in this process extracts of RAB and RC were extracted with boiling water with several mechanisms, including proliferation, migra- followed by ethanol, respectively. -

Dan Shen (Salvia Miltiorrhiza) in Medicine Xijun Yan Editor
Dan Shen (Salvia miltiorrhiza) in Medicine Xijun Yan Editor Dan Shen (Salvia miltiorrhiza) in Medicine Volume 3. Clinical Research 123 Editor Xijun Yan Tianjin China ISBN 978-94-017-9465-7 ISBN 978-94-017-9466-4 (eBook) DOI 10.1007/978-94-017-9466-4 Springer Dordrecht Heidelberg New York London Library of Congress Control Number: 2014950646 © Springer Science+Business Media Dordrecht and People’s Medical Publishing House 2015 This work is subject to copyright. All rights are reserved by the Publishers, whether the whole or part of the material is concerned, specifically the rights of translation, reprinting, reuse of illustrations, recitation, broadcasting, reproduction on microfilms or in any other physical way, and transmission or information storage and retrieval, electronic adaptation, computer software, or by similar or dissimilar methodology now known or hereafter developed. Exempted from this legal reservation are brief excerpts in connection with reviews or scholarly analysis or material supplied specifically for the purpose of being entered and executed on a computer system, for exclusive use by the purchaser of the work. Duplication of this publication or parts thereof is permitted only under the provisions of the Copyright Law of the Publishers’ locations, in its current version, and permission for use must always be obtained from Springer. Permissions for use may be obtained through RightsLink at the Copyright Clearance Center. Violations are liable to prosecution under the respective Copyright Law. The use of general descriptive names, registered names, trademarks, service marks, etc. in this publication does not imply, even in the absence of a specific statement, that such names are exempt from the relevant protective laws and regulations and therefore free for general use. -

Ethnomedicine Study on Traditional Medicinal Plants in the Wuliang
Gao et al. Journal of Ethnobiology and Ethnomedicine (2019) 15:41 https://doi.org/10.1186/s13002-019-0316-1 RESEARCH Open Access Ethnomedicine study on traditional medicinal plants in the Wuliang Mountains of Jingdong, Yunnan, China Lunlun Gao1,2,3†, Neng Wei3,4†, Guoping Yang1, Zhenxian Zhang1,3, Guizhou Liu1 and Chuantao Cai1* Abstract Background: The Wuliang Mountains of the Jingdong region is a settlement area of the Yi community located in south-western Yunnan Province in China. Due to its unique geographical location, this area harbours abundant medicinal plant resources. The medicinal plants used by the local people have a long history and play an important role in their daily life. During the long-term mixed lifestyle, the knowledge of traditional medicinal plants in different communities has been assimilated to some extent. Therefore, this paper is based on ethnobotanical investigations to document traditional medicinal plants used by local people and discuss the differences between the Yi and Han communities in the study area. Methods: Data on traditional medicinal plants were collected from September 2016 to August 2017 in the Yi autonomous county of Jingdong. Seven townships and 16 villages were selected for the field investigations. Information was obtained through key informant interviews. A total of 44 key informants were interviewed, and all of them were herbalists or herbal sellers. Results: In this study, a total of 302 traditional medicinal plant species belonging to 117 families and 252 genera were investigated and documented, most of which were obtained from herbalists. Although family Asteraceae was the most prevalent, with 27 species, the most commonly utilized species were members of family Papaveraceae, Dactylicapnos scandens (D. -

Antibacterial Activity of the Bioactive Fractions of Cyathula Uncinulata (Amaranthaceae)
European Journal of Medicinal Plants 10(1): 1-9, 2015, Article no.EJMP.18649 ISSN: 2231-0894 SCIENCEDOMAIN international www.sciencedomain.org Antibacterial Activity of the Bioactive Fractions of Cyathula uncinulata (Amaranthaceae) M. A. Bisi-Johnson1,2*, C. L. Obi3, T. Hattori4, Y. Oshima5, B. B. Samuel6 and J. N. Eloff6 1Department of Medical Microbiology, Walter Sisulu University, Mthatha 5117, South Africa. 2Department of Microbiology, Obafemi Awolowo University, Ile-Ife, Nigeria. 3Directorates of Academic Affairs, University of Fort Hare, Alice 5700, South Africa. 4Department of Emerging Infectious Diseases, School of Medicine, Postgraduate Division, Tohoku University, Sendai, Japan. 5Department of Natural Products Chemistry, Graduate School of Pharmaceutical Sciences, Tohoku University, Sendai, Japan. 6Department of Paraclinical Science, Phytomedicine Programme, Faculty of Veterinary Medicine, University of Pretoria, Onderstepoort 0110, South Africa. Authors’ contributions This work was carried out in collaboration between all authors with joint design of the study by authors MABJ and CLO. Author MABJ managed the literature searches, wrote the protocol, performed laboratory study, the statistical analysis and wrote the first draft of the manuscript. Authors CLO, JNE, TH, YO and BBS managed the analyses and supervision of the study. All authors read and approved the final manuscript. Article Information DOI: 10.9734/EJMP/2015/18649 Editor(s): (1) Marcello Iriti, Professor of Plant Biology and Pathology, Department of Agricultural and Environmental Sciences, Milan State University, Italy. Reviewers: (1) Hazem Mohammed Ebraheem Shaheen, Department of Pharmacology, Damanhour University, Egypt. (2) Rodrigo Crespo Mosca, São Paulo University, Brazil. Complete Peer review History: http://sciencedomain.org/review-history/10185 Received 4th May 2015 th Original Research Article Accepted 29 May 2015 Published 14th July 2015 ABSTRACT Cyathula uncinulata (Schrad.) Schinz is used in ethnomedicine in various parts of the world.