Beige Adipose Tissue Activities in Human Obesity
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

RAL COLOR CHART ***** This Chart Is to Be Used As a Guide Only. Colors May Appear Slightly Different ***** Green Beige Purple V
RAL COLOR CHART ***** This Chart is to be used as a guide only. Colors May Appear Slightly Different ***** RAL 1000 Green Beige RAL 4007 Purple Violet RAL 7008 Khaki Grey RAL 4008 RAL 7009 RAL 1001 Beige Signal Violet Green Grey Tarpaulin RAL 1002 Sand Yellow RAL 4009 Pastel Violet RAL 7010 Grey RAL 1003 Signal Yellow RAL 5000 Violet Blue RAL 7011 Iron Grey RAL 1004 Golden Yellow RAL 5001 Green Blue RAL 7012 Basalt Grey Ultramarine RAL 1005 Honey Yellow RAL 5002 RAL 7013 Brown Grey Blue RAL 1006 Maize Yellow RAL 5003 Saphire Blue RAL 7015 Slate Grey Anthracite RAL 1007 Chrome Yellow RAL 5004 Black Blue RAL 7016 Grey RAL 1011 Brown Beige RAL 5005 Signal Blue RAL 7021 Black Grey RAL 1012 Lemon Yellow RAL 5007 Brillant Blue RAL 7022 Umbra Grey Concrete RAL 1013 Oyster White RAL 5008 Grey Blue RAL 7023 Grey Graphite RAL 1014 Ivory RAL 5009 Azure Blue RAL 7024 Grey Granite RAL 1015 Light Ivory RAL 5010 Gentian Blue RAL 7026 Grey RAL 1016 Sulfer Yellow RAL 5011 Steel Blue RAL 7030 Stone Grey RAL 1017 Saffron Yellow RAL 5012 Light Blue RAL 7031 Blue Grey RAL 1018 Zinc Yellow RAL 5013 Cobolt Blue RAL 7032 Pebble Grey Cement RAL 1019 Grey Beige RAL 5014 Pigieon Blue RAL 7033 Grey RAL 1020 Olive Yellow RAL 5015 Sky Blue RAL 7034 Yellow Grey RAL 1021 Rape Yellow RAL 5017 Traffic Blue RAL 7035 Light Grey Platinum RAL 1023 Traffic Yellow RAL 5018 Turquiose Blue RAL 7036 Grey RAL 1024 Ochre Yellow RAL 5019 Capri Blue RAL 7037 Dusty Grey RAL 1027 Curry RAL 5020 Ocean Blue RAL 7038 Agate Grey RAL 1028 Melon Yellow RAL 5021 Water Blue RAL 7039 Quartz Grey -

Black, Brown and Beige
Jazz Lines Publications Presents black, brown, and beige by duke ellington prepared for Publication by dylan canterbury, Rob DuBoff, and Jeffrey Sultanof complete full score jlp-7366 By Duke Ellington Copyright © 1946 (Renewed) by G. Schirmer, Inc. (ASCAP) International Copyright Secured. All Rights Reserved. Reprinted by Permission. Logos, Graphics, and Layout Copyright © 2017 The Jazz Lines Foundation Inc. Published by the Jazz Lines Foundation Inc., a not-for-profit jazz research organization dedicated to preserving and promoting America’s musical heritage. The Jazz Lines Foundation Inc. PO Box 1236 Saratoga Springs NY 12866 USA duke ellington series black, brown, and beige (1943) Biographies: Edward Kennedy ‘Duke’ Ellington influenced millions of people both around the world and at home. In his fifty-year career he played over 20,000 performances in Europe, Latin America, the Middle East as well as Asia. Simply put, Ellington transcends boundaries and fills the world with a treasure trove of music that renews itself through every generation of fans and music-lovers. His legacy continues to live onward and will endure for generations to come. Wynton Marsalis said it best when he said, “His music sounds like America.” Because of the unmatched artistic heights to which he soared, no one deserves the phrase “beyond category” more than Ellington, for it aptly describes his life as well. When asked what inspired him to write, Ellington replied, “My men and my race are the inspiration of my work. I try to catch the character and mood and feeling of my people.” Duke Ellington is best remembered for the over 3,000 songs that he composed during his lifetime. -

Gametime Color Options
www.gametime.com GameTime ... Color Options KidTime® Color Options Deck Colors WallCano® Handholds Plastic Colors Metal Colors Dark Green Red Yellow Red Yellow Butterscotch Blue Red Orange Green Red Royal Purple New! Beige Burgundy Blue Primary Tempo Natural Brown Blue New! Thermoplastic deck coating only available in brown. Net Colors Timber Décor Colors Special Rock Colors Royal Purple Freestanding Net Climbers Xscape Nets Pyramid Nets Redwood Sky Blue Sandstone Blue New! Deep Granite Spring Green Red Rock Sky Blue (RockScape only) New! Spring Green Green Red Blue Green Black Red Black Red Cedar Green ™ Polyethylene Colors (HDPE) SunBlox Canopy & Shade Colors Brown Dark Green Sunflower Yellow Red Royal Blue Laguna Blue Red Red/Yellow Red/White New! Beige Brown Yellow/Red Yellow/Black New! Yellow Navy Blue Turquoise Rain Forest Terra Cotta Beige Meadow Green/Beige Green/White New! Green New! Metallic Earth Blue/Beige Blue/White Arizona Silver Black White Blue New! New! New Eco Colors, Black Earth, Meadow & Stone contain Beige/Green Black/White Stone recycled plastic Beige causing unique White New! color variation. New! Colors shown are approximate, ask your representative to view current color samples. ® GameTime Play Palette Color Schemes Play Palette Color Schemes Play Palettes The easy way to pick colors Periwinkle Delightful Fresh Blue Blue Beige Our color experts have years of experience Plastic Plastic Plastic choosing the right color for each component to blend harmoniously into an overall palette. They’ve Butterscotch Spring Green Green selected 15 great combinations for you that take Uprights Uprights Uprights the guesswork out of choosing colors, whether Butterscotch Burgundy Spring Green you want a bright, subdued, or natural look. -

Color Chart Colorchart
Color Chart AMERICANA ACRYLICS Snow (Titanium) White White Wash Cool White Warm White Light Buttermilk Buttermilk Oyster Beige Antique White Desert Sand Bleached Sand Eggshell Pink Chiffon Baby Blush Cotton Candy Electric Pink Poodleskirt Pink Baby Pink Petal Pink Bubblegum Pink Carousel Pink Royal Fuchsia Wild Berry Peony Pink Boysenberry Pink Dragon Fruit Joyful Pink Razzle Berry Berry Cobbler French Mauve Vintage Pink Terra Coral Blush Pink Coral Scarlet Watermelon Slice Cadmium Red Red Alert Cinnamon Drop True Red Calico Red Cherry Red Tuscan Red Berry Red Santa Red Brilliant Red Primary Red Country Red Tomato Red Naphthol Red Oxblood Burgundy Wine Heritage Brick Alizarin Crimson Deep Burgundy Napa Red Rookwood Red Antique Maroon Mulberry Cranberry Wine Natural Buff Sugared Peach White Peach Warm Beige Coral Cloud Cactus Flower Melon Coral Blush Bright Salmon Peaches 'n Cream Coral Shell Tangerine Bright Orange Jack-O'-Lantern Orange Spiced Pumpkin Tangelo Orange Orange Flame Canyon Orange Warm Sunset Cadmium Orange Dried Clay Persimmon Burnt Orange Georgia Clay Banana Cream Sand Pineapple Sunny Day Lemon Yellow Summer Squash Bright Yellow Cadmium Yellow Yellow Light Golden Yellow Primary Yellow Saffron Yellow Moon Yellow Marigold Golden Straw Yellow Ochre Camel True Ochre Antique Gold Antique Gold Deep Citron Green Margarita Chartreuse Yellow Olive Green Yellow Green Matcha Green Wasabi Green Celery Shoot Antique Green Light Sage Light Lime Pistachio Mint Irish Moss Sweet Mint Sage Mint Mint Julep Green Jadeite Glass Green Tree Jade -

Green Beige Beige Sand Yellow Signal Yellow Golden Yellow Honey Yellow Maize Yellow Daffodil Yellow Brown Beige Lemon Yellow
GREEN BEIGE BEIGE SAND YELLOW SIGNAL YELLOW GOLDEN YELLOW DAFFODIL HONEY YELLOW MAIZE YELLOW YELLOW BROWN BEIGE LEMON YELLOW OYSTER WHITE IVORY LIGHT IVORY SULFUR YELLOW SAFFRON YELLOW ZINC YELLOW GREY BEIGE OLIVE YELLOW COLZA YELLOW TRAFFIC YELLOW LUMINOUS OCHRE YELLOW YELLOW CURRY MELON YELLOW BROOM YELLOW DAHLIA YELLOW PASTEL YELLOW PEARL BEIGE PEARL GOLD SUN YELLOW YELLOW ORANGE RED ORANGE VERMILION PASTEL ORANGE PURE ORANGE LUMINOUS LUMINOUS BRIGHT RED ORANGE BRIGHT ORANGE ORANGE TRAFFIC ORANGE SIGNAL ORANGE DEEP ORANGE SALMON ORANGE PEARL ORANGE FLAME RED SIGNAL RED CARMINE RED RUBY RED PURPLE RED WINE RED BLACK RED OXIDE RED BROWN RED BEIGE RED TOMATO RED ANTIQUE PINK LIGHT PINK CORAL RED ROSE LUMINOUS STRAWBERRY RED TRAFFIC RED SALMON PINK LUMINOUS RED BRIGHT RED RASPBERRY RED PURE RED ORIENT RED PEARL RUBY RED PEARL PINK RED LILAC RED VIOLET HEATHER VIOLET CLARET VIOLET BLUE LILAC TRAFFIC PURPLE PURPLE VIOLET SIGNAL VIOLET PASTEL VIOLET TELEMAGENTA PEARL BLACK PEARL VIOLET BERRY ULTRAMARINE VIOLET BLUE GREEN BLUE BLUE SAPHIRE BLUE BLACK BLUE SIGNAL BLUE BRILLANT BLUE GREY BLUE AZURE BLUE GENTIAN BLUE STEEL BLUE LIGHT BLUE COBALT BLUE PIGEON BLUE SKY BLUE TRAFFIC BLUE TURQUOISE BLUE CAPRI BLUE OCEAN BLUE WATER BLUE PEARL GENTIAN PEARL NIGHT NIGHT BLUE DISTANT BLUE PASTEL BLUE BLUE BLUE PATINA GREEN EMERALD GREEN LEAF GREEN OLIVE GREEN BLUE GREEN MOSS GREEN GREY OLIVE BOTTLE GREEN BROWN GREEN FIR GREEN GRASS GREEN RESEDA GREEN BLACK GREEN REED GREEN YELLOW OLIVE TURQUOISE BLACK OLIVE GREEN MAY GREEN YELLOW GREEN PASTEL GREEN CHROME -
Dsb Color Chart 2020
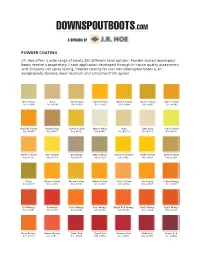
POWDER COATING J.R. Hoe offers a wide range of nearly 200 different color options. Powder coated downspout boots receive a proprietary 2-coat application developed through in-house quality assessment and 3rd party salt spray testing. Powder coating for cast iron downspout boots is an exceptionally durable, wear-resistant and attractive finish option. Green Beige Beige Sand Yellow Signal Yellow Golden Yellow Honey Yellow Maize Yellow RAL #1000 RAL #1001 RAL #1002 RAL #1003 RAL #1004 RAL #1005 RAL #1006 Daffodil Yellow Brown Beige Lemon Yellow Oyster White Ivory Light Ivory Sulfur Yellow RAL #1007 RAL #1011 RAL #1012 RAL #1013 RAL #1014 RAL #1015 RAL #1016 Saffron Yellow Zinc Yelllow Grey Beige Olive Yellow Rapeseed Yellow Traffic Yellow Ochre Yellow RAL #1017 RAL #1018 RAL #1019 RAL #1020 RAL #1021 RAL #1023 RAL #1024 Curry Melon Yelllow Broom Yellow Dahlia Yellow Pastel Yellow Sun Yellow Yellow Orange RAL #1027 RAL #1028 RAL #1032 RAL #1033 RAL #1034 RAL #1037 RAL #2000 Red Orange Vermillion Pastel Orange Pure Orange Bright Red Orange Traffic Orange Signal Orange RAL #2001 RAL #2002 RAL #2003 RAL #2004 RAL #2008 RAL #2009 RAL #2010 Deep Orange Salmon Orange Flame Red Signal Red Carmine Red Ruby Red Purple Red RAL #2011 RAL #2012 RAL #3000 RAL #3001 RAL #3002 RAL #3003 RAL #3004 Wine Red Black Red Oxide Red Brown Red Beige Red Tomato Red Antique Rose RAL #3005 RAL #3007 RAL #3009 RAL #3011 RAL #3012 RAL #3013 RAL #3014 Light Pink Coral Red Rose Strawberry Red Traffic Red Salmon Pink Raspberry Red RAL #3015 RAL #3016 RAL #3017 RAL #3018 RAL #3020 -

Urethane Color Chart
ARCHITECTURAL URETHANE SEALANTS Architectural Weatherproofing Products ARCHITECTURAL URETHANEARCHITECTURAL SEALANTS2-PART Architectural Weatherproofing Products Dynatrol™ II | Dynaflex | Dynatred™ URETHANE URETHANE SEALANTS2-PART Architectural Weatherproofing Products Dynatrol™ II | Dynaflex | Dynatred™ URETHANE BRITE WHITE CF26 TAN 545 STANDARD 2-PART Dynatrol™ II | Dynaflex | Dynatred™ URETHANE COLORSTANDARD TRU-WHITEBRITE WHITE CF26345 BUFFTAN 545512 BRITE WHITE CF26 TAN 545 GUIDECOLORSTANDARD OFF-WHITETRU-WHITE 345516 COLONIALBUFF TAN CF13512 TRU-WHITE 345 BUFF 512 GUIDECOLOR DOVEROFF-WHITE SKY CF14516 MOCHACOLONIAL CREAM TAN CF34CF13 OFF-WHITE COLONIAL TAN DOVER SKY CF14516 MOCHA CREAM CF34CF13 GUIDECustom colors available ANODIZED ALUMINUM 804 TOASTED ALMOND CF54 upon request. DOVER SKY CF14 MOCHA CREAM CF34 Custom colors available BRUSHEDANODIZED PEWTER ALUMINUM CF42804 NATURALTOASTED ALMONDSTONE CF54565 upon request. ANODIZED ALUMINUM 804 TOASTED ALMOND CF54 Custom colors available ALUMINUMBRUSHED PEWTER STONE CF42515 DESERTNATURAL TAN STONE 565530 upon request. BRUSHED PEWTER CF42 NATURAL STONE 565 Color Packs for Standard STONEALUMINUM GREY STONE CF53515 ADOBEDESERT ACCENT TAN CF10530 and Non- Standard ALUMINUM STONE 515 DESERT TAN 530 ColorsColor Packs are sold for inStandard 5 unit LONDONSTONE GREY FOG CF53CF44 REDWOODADOBE ACCENT TAN CF43CF10 increments.and Non- Standard Color Packs for Standard STONE GREY CF53 ADOBE ACCENT CF10 Colors are sold in 5 unit DARKLONDON GRAY FOG CF44048 BRICKREDWOOD RED TAN CF43CF16 increments.and -

Empire Collection Rugs
Empire Collection Wool Colors Page 1 of 6 W500 W535 W210 W405 W502 W536 White Almond Vanilla Ice Cream Angora Beige W728 W537 W578 W735 W750 W400 Corn Amber Brown Powder Sand Haze Latte Mocha W751 W752 W542 W713 W503 W508 Pastel Rose Beige Beaver Taupe Oat Walnut W509 W510 W546 W512 W513 W514 Dark Beige Bronze Chamomile Vanilla Sahara Honey Mustard W552 W994 W213 W202 W529 W753 Olive Tree Linseed Fog Beige Nugget Gold Deep Gold Tender Yellow W940 W715 W487 W215 W527 W714 Yellow Cream Wheat Empire Yellow Lemon Yellow Dark Yellow empirecollectionrugs.com Empire Collection Wool Colors Page 2 of 6 W732 W540 W541 W700 W520 W521 Olive Honey Light Brown Brown Green Golden Brown Cinnamon W523 W211 W524 W216 W525 W506 Brown Friar Brown Tobacco Bitter Chocolate Dark Brown Chocolate W665 W539 W544 W663 W680 W200 Vintage Rose Mahogany Rust Tile Red Ferrari W679 W755 W681 W674 W675 W677 Tomato Rouge Blood Dark Red Ruby Dark Aubergine W682 W754 W689 W690 W684 W688 Apricot Saron Gingery Fog Dark Orange Vibrant Orange W683 W686 W687 W744 W678 W661 Pumpkin Light Orange Tangerine Flame Orange Chili empirecollectionrugs.com Empire Collection Wool Colors Page 3 of 6 W212 W668 W698 W711 W545 W659 Nude Light Peach Potpourri Mellow Rose Terracotta Berry Rose W660 W214 W201 W654 W673 W991 Maroon Apricot Blush Coral Pink Pink Cherry Electric Pink W756 W694 W229 W757 W701 W226 Light Pink Crystal Pink Crystal Rose Rose Pink Rose Virtual Rose W736 W745 W716 W699 W717 W399 Virtual Pink Fuchsia Cashmere Rose Purple Rose Purple Vintage Porto W209 W758 W630 W709 W759 -

Beige Book Summary of Commentary on Current Economic Conditions by Federal Reserve District
For use at 2:00 PM EDT Wednesday April 14, 2021 TheBeigeBook Summary of Commentary on Current Economic Conditions By Federal Reserve District April 2021 Federal Reserve Districts Minneapolis Boston New York Chicago Cleveland Philadelphia San Francisco Kansas City St. Louis Richmond Atlanta Dallas Alaska and Hawaii The System serves commonwealths and territories as follows: the New York Bank serves the are part of the Commonwealth of Puerto Rico and the U.S. Virgin Islands; the San Francisco Bank serves San Francisco District. American Samoa, Guam, and the Commonwealth of the Northern Mariana Islands. This report was prepared at the Federal Reserve Bank of Dallas based on information collected on or before April 5, 2021. This document summarizes comments received from contacts outside the Feder‐ al Reserve System and is not a commentary on the views of Federal Reserve officials. National Summary 1 What is the Beige Book? The Beige Book is a Federal Reserve System publication about current economic conditions across the 12 Federal Reserve Districts. It charac- Boston A-1 terizes regional economic conditions and prospects based on a variety First District of mostly qualitative information, gathered directly from each District’s sources. Reports are published eight times per year. New York B-1 What is the purpose of the Beige Book? Second District The Beige Book is intended to characterize the change in economic conditions since the last report. Outreach for the Beige Book is one of Philadelphia C-1 many ways the Federal Reserve System engages with businesses and Third District other organizations about economic developments in their communi- ties. -

COLONIAL GEORGIAN 1700-1776 FEDERAL 1780-1820 Base Color Trim Color Door Color Brick White Black Off-White Buff Natural Pale Ye
COLONIAL GEORGIAN 1700-1776 Base Color Trim Color Door Color Natural Same as Base Dark Brown Spanish Brown (dark, dul red) White Black/Green Prussian (Dark Blue/Green) Dark Grey Indian Red ("verging on scarlet") Dark Red Yellow Orche Green FEDERAL 1780-1820 Base Color Trim Color Door Color Brick White Black Off-White Buff Natural Pale Yellow Medium Blue Brown Ochre White Pale Yellow White Red Soft Beige Pale Green Medium Grey Medium Blue GOTHIC REVIVAL 1850-1870 TO EARLY VICTORIAN Base Color Trim Color Door Color Shades of Grey Darker than Base Unpainted Wood Drab or Fawn Darker than Base Oak Sage Lighter than Base Straw/Sand Lighter than Base Chocolate Red Buff Dark Grey Brick Pink Dark Green/Brown Mustard Straw Colored Stucco BRACKETED OR ITALIANATE 1840-1880 Base Color Trim Color Door Color Pale Beige Darker Beige Black Golden Sand Lighter Sand Natural Golden Brown Darker Brown Burgundy Olive Branch Stain Lighter Olive Light Grey Dark Grey Deep Grey Light Grey Grey Stain Lighter Stain Yellow Orche Dark Green Blue Grey Medium Brown Stone Dark Brown Old Gold Medium Red Fawn Sash (Reddish Brown) Buff Shutter Green MANSARD or SECOND EMPIRE 1855-1885 Base Color Trim Color Door Color Pale Olive Ivory Olive Rose Pale Rose Dark Green Peach Pale Peach Golden Sands Stain Ivory/Yellow Sash Olive Tan Bittersweet Straw Cream/Yellow Sash Light Yellow White/Brown Brown Brown/Bittersweet Sash & Shutters Light Brownstone Medium Brownstone QUEEN ANNE 1875-1905 MULTICOLORED PERIOD Base Color Trim Color Door Color Light Olive Dark Olive/Dark Red Accent -

Technical Section
Color Codes Charts 8 & 9 CHART 8: DIN 47100 (without color repetition) (For telephone and electronic use only) UNITRONIC® FD CY, UNITRONIC® FD CP, UNITRONIC® FD 890, UNITRONIC® LIYY, UNITRONIC® LIYCY, UNITRONIC® LIFYCY Conductor Color Conductor Color Conductor Color Conductor Color 1 White 17 White/Gray 33 Green/Red 49 White/Green/Black 2 Brown 18 Gray/Brown 34 Yellow/Red 50 Brown/Green/Black 3 Green 19 White/Pink 35 Green/Black 51 White/Yellow/Black 4 Yellow 20 Pink/Brown 36 Yellow/Black 52 Yellow/Brown/Black 5 Gray 21 White/Blue 37 Gray/Blue 53 White/Gray/Black 6 Pink 22 Brown/Blue 38 Pink/Blue 54 Gray/Brown/Black 7 Blue 23 White/Red 39 Gray/Red 55 White/Pink/Black 8 Red 24 Brown/Red 40 Pink/Red 56 Pink/Brown/Black 9 Black 25 White/Black 41 Gray/Black 57 White/Blue/Black 10 Violet 26 Brown/Black 42 Pink/Black 58 Brown/Blue/Black 11 Gray/Pink 27 Gray/Green 43 Blue/Black 59 White/Red/Black 12 Red/Blue 28 Yellow/Gray 44 Red/Black 60 Brown/Red/Black 13 White/Green 29 Pink/Green 45 White/Brown/Black 61 Black/White 14 Brown/Green 30 Yellow/Pink 46 Yellow/Green/Black 15 White/Yellow 31 Green/Blue 47 Gray/Pink/Black 16 Yellow/Brown 32 Yellow/Blue 48 Red/Blue/Black CHART 9: Color Code for 6 or More Conductors OLFLEX® 100, 100 CY, 100 SY Conductor Color Conductor Color Conductor Color Conductor Color 0 Green/Yellow 26 Violet/Black 52 Trans/Red 78 Beige/White/Blue 1 White 27 Pink/Black 53 Beige/Red 79 Gray/White/Brown 2 Black 28 Orange/Black 54 Pink/Violet 80 Red/White/Brown 3 Blue 29 Trans/Black 55 Orange/Violet 81 Violet/White/Brown 4 Brown -

Classico Limewash Is Different Than Any Classico Limewash Is Available In: Other Paint Or Process
© CLASSICO CLASSICO LIMEWASH IS DIFFERENT THAN ANY CLASSICO LIMEWASH IS AVAILABLE IN: OTHER PAINT OR PROCESS. (COVERS 120 SQ/FT) Whitewashed brick exteriors are gorgeous, and have a charming Old World feel. They have a 1 QT. MAKES 1.5 - 2 QT. AFTER DILUTION LIMEWASH freshly painted look, but because some of the brick remains exposed, the warmth and quality of it PERFECT FOR INTERIOR FIREPLACES! endures. In Europe, the iconic whitewash effect was created over hundreds of years of traditional limewash application, but now you can achieve this look naturally and beautifully overnight. (COVERS 300 SQ/FT) .67 GAL. MAKES 1 - 1.5 GAL. AFTER DILUTION Classico Limewash is aged like a fine wine, slaked, and then specially formulated to be applied (COVERS 1800 SQ/FT) like traditional paint. Different than any other paint or white wash process, it is an authentic 4 GAL. MAKES 6 - 8 GAL. AFTER DILUTION slaked-lime paint that can instantly be washed off to create the exposed brick look you want. Unlike acrylic paint, or the German smear technique using mortar, it is made to last on the exterior and calcifies to a rock solid finish so it won’t peel, chip, or flake off with little TIPS FOR APPLICATION: maintenance. Now you can transform your home to the look you want in an easy, one coat application and wash off process. • Watch the how-to videos at romabio.com/limewash to see how easy it is. • Check for absorbency on your masonry surface with water to see if it soaks up CLASSICO LIMEWASH IS SPECIALLY FORMULATED (absorbent), and doesn’t sit directly on the surface (non-absorbent and will not work).