Double Superior Vena Cava and Anomaly of Cardiovascular System with a Review of the Literature
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

MDCT of Interatrial Septum
Diagnostic and Interventional Imaging (2015) 96, 891—899 PICTORIAL REVIEW /Cardiovascular imaging MDCT of interatrial septum ∗ D. Yasunaga , M. Hamon Service de radiologie, pôle d’imagerie, CHU de Caen, avenue de la Côte-de-Nacre, 14033 Caen Cedex 9, France KEYWORDS Abstract ECG-gated cardiac multidetector row computed tomography (MDCT) allows precise Cardiac CT; analysis of the interatrial septum (IAS). This pictorial review provides a detailed description of Interatrial septum; the normal anatomy, variants and abnormalities of the IAS such as patent foramen ovale, con- Patent foramen genital abnormalities such as atrial septal defects as well as tumors and tumoral-like processes ovale; that develop on the IAS. Secundum ASD © 2015 Published by Elsevier Masson SAS on behalf of the Éditions françaises de radiologie. Introduction Major technical advances in computed tomography (CT) in recent years have made it pos- sible to use multidetector row CT (MDCT) in the field of cardiac imaging. Besides coronary arteries, ECG-gated cardiac MDCT provides high-resolution images of all cardiac structures. It is therefore important for radiologists to understand and be able to analyze the normal anatomical structures, variants and diseases of these different structures. This article provides an analysis of the interatrial septum (IAS) based on a pictorial review. After a short embryological and anatomical description, we will illustrate the nor- mal anatomy and variants of the IAS, anomalies such as patent foramen ovale (PFO), congenital diseases such as atrial septal defects (ASD) as well as tumors and tumoral-like processes that develop on the IAS. Abbreviations: ASA, atrial septal aneurysm; ASD, atrial septal defect; ECG, electrocardiogram; IAS, interatrial septum; IVC, inferior vena cava; IVS, interventricular septum; LV, left ventricle; M, myxoma; PFO, patent foramen ovale; RSPV, right superior pulmonary vein; RV, right ventricle; SVC, superior vena cava; MIP, maximal intensity projection; TEE, transesophageal echocardiography; TV, tricuspid valve. -

Cardiovascular System Note: the Cardiovascular System Develops Early (Week 3), Enabling the Embryo to Grow Beyond the Short
Lymphatics: Lymph vessel formation is similar to blood angiogenesis. Lymphatics begin as lymph sacs in three regions: jugular (near brachiocephalic veins); cranial abdominal (future cysterna chyla); and iliac region. Lym- phatic vessels (ducts) form as outgrowths of the sacs. mesenchyme Lymph nodes are produced by localized mesoder- sinusoid lymph duct lumen mal invaginations that partition the vessel lumen into sinu- soids. The mesoderm develops a reticular framework within which mesodermal lymphocytes accumulate. The spleen and hemal nodes (in ruminants) invagination develop similar to the way lymph nodes develop. Lymph Node Formation Prior to birth, fetal circulation is designed for an in utero aqueous environment where the pla- centa oxygenates fetal blood. Suddenly, at birth... Three In-Utero Adjustments ductus Stretching and constriction of arteriosus umbilical arteries shifts fetal blood flow aortic arch from the placenta to the fetus. Reduced pulmonary trunk L atrium venous return through the (left) umbili- foramen ovale R cal vein and ductus venosus allows the atrium latter to gradually close (over a period caudal vena cava of days). Bradykinin released by expand- ductus venosus ing lungs and increased oxygen concen- tration in blood triggers constriction of aorta the ductus arteriosus which, over two liver months, is gradually converted to a fibrous structure, the ligamentum arte- umbilical v. riosum. portal v. The increased blood flow to the lungs and then to the left atrium equalizes pres- sure in the two atria, resulting in closure umbilical aa. of the foramen ovale that eventually grows permanent. 29 The cardiogenic area, the place where the embryonic heart originates, is located . -

Endothelial Dysfunction May Link Interatrial Septal Abnormalities and MTHFR-Inherited Defects to Cryptogenic Stroke Predisposition
biomolecules Article Endothelial Dysfunction May Link Interatrial Septal Abnormalities and MTHFR-Inherited Defects to Cryptogenic Stroke Predisposition 1, 2, 1 1 Luca Sgarra y, Alessandro Santo Bortone y, Maria Assunta Potenza , Carmela Nacci , Maria Antonietta De Salvia 1, Tommaso Acquaviva 2, Emanuela De Cillis 2, Marco Matteo Ciccone 2, Massimo Grimaldi 3 and Monica Montagnani 1,* 1 Department of Biomedical Sciences and Human Oncology—Section of Pharmacology, Medical School, University of Bari “Aldo Moro”, 70124 Bari, Italy; [email protected] (L.S.); [email protected] (M.A.P.); [email protected] (C.N.); [email protected] (M.A.D.S.) 2 Department of Emergency and Organ Transplantation—Section of Cardiovascular Diseases, Medical School, University of Bari “Aldo Moro”, 70124 Bari, Italy; [email protected] (A.S.B.); [email protected] (T.A.); [email protected] (E.D.C.); [email protected] (M.M.C.) 3 General Hospital “F. Miulli” Acquaviva delle Fonti, 70021 Bari, Italy; fi[email protected] * Correspondence: [email protected] These authors contributed equally to this work. y Received: 28 March 2020; Accepted: 2 June 2020; Published: 4 June 2020 Abstract: We explored the significance of the L-Arginine/asymmetric dimethylarginine (L-Arg/ADMA) ratio as a biomarker of endothelial dysfunction in stroke patients. To this aim, we evaluated the correlation, in terms of severity, between the degree of endothelial dysfunction (by L-Arg/ADMA ratio), the methylene tetrahydrofolate reductase (MTHFR) genotype, and the interatrial septum (IAS) phenotype in subject with a history of stroke. Methods and Results: L-Arg, ADMA, and MTHFR genotypes were evaluated; the IAS phenotype was assessed by transesophageal echocardiography. -

The Interventricular Septum by E
Thorax: first published as 10.1136/thx.12.4.304 on 1 December 1957. Downloaded from Thorax (1957), 12, 304. THE INTERVENTRICULAR SEPTUM BY E. W. T. MORRIS From the Anatomy Department, St. Thomas's Hospital Medical School, Londoni (RECEIVED FOR PUBLICATION JULY 26, 1957) It is difficult to find in the literature a clear and between the tips of its two horns where the concise account of the development and form of boundary is formed by the fused atrioventricular the interventricular septum. Moreover, some of cushion (A, in Fig. 4). This septum does not lie the accounts in the clinical literature are at vari- in one plane and the main part of its free border ance with that generally accepted by embryo- forms a spiral (Figs. 4 and 5). logists. For this reason and in view of the recent (2) While the muscular part is forming. changes technical advances in the surgery of the heart, it are taking place in the relative positions of the seems opportune to describe the development and bulbus cordis and the ventricles. Earlier the heart anatomy of the interventricular septum and to tube is flexed at the bulboventricular junction so correlate this knowledge as far as possible with that the bulbus cordis comes to lie ventrally and the sites of interventricular septal defects. to the right of the ventricle (Fig. 2). Their con- At an early stage the heart consists of the sinus tiguous walls form a septum-the bulboven- venosus, the common atrium, the common ven- tricular septum-around the lower free border tricle, and the bulbus cordis, serially arranged in of which the two cavities communicate (see Figs.copyright. -
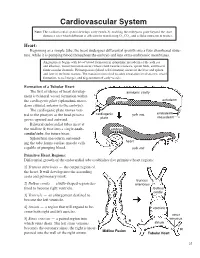
Cardiovascular System Note: the Cardiovascular System Develops Early (Week-3), Enabling the Embryo to Grow Beyond the Short
Cardiovascular System Note: The cardiovascular system develops early (week-3), enabling the embryo to grow beyond the short distances over which diffusion is efficient for transferring 2O , CO2, and cellular nutrients & wastes. Heart: Beginning as a simple tube, the heart undergoes differential growth into a four chambered struc- ture, while it is pumping blood throughout the embryo and into extra-embryonic membranes. Angiogenesis begins with blood island formation in splanchnic mesoderm of the yolk sac and allantois. Vessel formation occurs when island vesicles coalesce, sprout buds, and fuse to form vascular channels. Hematopoiesis (blood cell formation) occurs in the liver and spleen and later in the bone marrow. The transition from fetal to adult circulation involves new vessel formation, vessel merger, and degeneration of early vessels. Formation of a Tubular Heart: The first evidence of heart develop- amnionic cavity ment is bilateral vessel formation within ectoderm the cardiogenic plate (splanchnic meso- embryo derm situated anterior to the embryo). The cardiogenic plate moves ven- tral to the pharynx as the head process cardiogenic yolk sac endoderm mesoderm grows upward and outward. plate Bilateral endocardial tubes meet at the midline & fuse into a single endo- embryo cardial tube, the future heart. Splanchnic mesoderm surround- ing the tube forms cardiac muscle cells heart capable of pumping blood. yolk sac Primitive Heart Regions: Differential growth of the endocardial tube establishes five primitive heart regions: 1] Truncus arteriosus — the output region of the heart. It will develop into the ascending aorta and pulmonary trunk. truncus 2] Bulbus cordis — a bulb-shaped region des- arteriosus tined to become right ventricle. -

Atrial-Myxomas.Pdf
Atrial myxomas Atrial myxomas are the most common primary heart tumors. They are benign intracavitary cardiac neoplasms and accounting for one-third to one-half of cases at postmortem and for about three quarter of tumors treated surgically (Braunwald 2001). Because of nonspecific symptoms, early diagnosis may be a challenge. Left atrial myxoma may or may not produce characteristic findings on auscultation. Most atrial myxomas, whether left or right, arise from the atrial septum, usually from the region of the limbus of fossa ovalis. About 10% have other sites of origin, particularly posterior wall, anterior wall and the appendages (in order of frequency) (McAllister 1979). (Two- dimensional echocardiography is the diagnostic procedure of choice. Most atrial myxomas are benign and can be removed by surgical resection. Pathophysiology Myxomas account for 40-50% of primary cardiac tumors. Approximately 90% are solitary and pedunculated, and 75-85% occur in the left atrial cavity. Up to 25% of cases are found in the right atrium. Most cases are sporadic. Approximately 10% are familial and are transmitted in an autosomal dominant mode. Multiple tumors occur in approximately 50% of familial cases and are more frequently located in the ventricle (13% vs 2% in sporadic cases). Myxomas are polypoid, round, or oval. They are gelatinous with a smooth or lobulated surface and usually are white, yellowish, or brown such as of the figures 1 and 2 Fig. 1 Pathology specimens of multiple small myxomas resected from the right atrial surface. Fig. 2 Pathology specimen of myxoma from the vicinity of the interatrial groove, after near-total resection. -

The Sinus Venosus Typeof Interatrial Septal Defect*
Thorax: first published as 10.1136/thx.13.1.12 on 1 March 1958. Downloaded from Thorax (I9%8), 13, 12. THE SINUS VENOSUS TYPE OF INTERATRIAL SEPTAL DEFECT* BY H. R. S. HARLEY Cardiff (RECEIVED FOR PUBLICATION DECEMBER 30, 1957) Defects of the interatrial septum, other than namely, (1) it lies above and independent of valvular patency of the foramen ovale, are often the fossa ovalis; (2) its margin is incomplete, classified into ostium primum and ostium secun- being absent superiorly and incomplete pos- dum varieties. The relationship of the former type teriorly; and (3) it is associated with anomalous to abnormal development of the atrioventricular drainage of the right superior, and sometimes of canal has been stressed by several workers, includ- the right middle or inferior, pulmonary vein. This ing Rogers and Edwards (1948), Wakai and type of defect is illustrated in Fig. 1 (after Lewis Edwards (1956), Wakai, Swan, and Wood (1956), et al., 1955) and Fig. 2 (after Geddes, 1912). In Brandenburg and DuShane (1956), Toscano- the case reported by Ross (1956), who kindly per- Barbosa, Brandenburg, and Burchell (1956), and mitted me to see the heart, the interatrial Cooley and Kirklin (1956). These workers prefer communication was described as ". lying the term "persistent common within the orifice of atrioventricular the superior vena cava in itscopyright. canal " to "persistent ostium primum." medial wall opposite the mouths of the anomalous In addition to the above types of interatrial pulmonary veins." Ross goes on to say: "On septal defect there is a third variety, which was casual inspection of the interior of the left atrium, described as long ago as 1868 by Wagstaffe, but the defect was not visible unless a search was made which has come into prominence only since the within the superior caval orifice." The relation- http://thorax.bmj.com/ introduction of surgical repair of interatrial ship of the defect to the orifice of the superior communications under direct vision. -

Original Articles
Artigo Original %DVHV0RUIROyJLFDVSDUDR(VWXGRGR6HSWR,QWHUDWULDOQR)HWR Humano Morphological Basis for the Study of the Interatrial Septum in the Human Fetus Hugo Becker Amaral, Paulo Zielinsky, Aron Ferreira da Silveira, Ijoni Costabeber, Luiz Henrique Nicoloso, Olmiro Cezimbra de Souza Filho, Marcelo Salum, João Luiz Manica, Juliana Silveira Zanettini, Ane Micheli Costabeber 8QLGDGHGH&DUGLRORJLD)HWDOGR,QVWLWXWRGH&DUGLRORJLDGR5LR*UDQGHGR6XO'HSDUWDPHQWRGH0RUIRORJLDGR&HQWURGH&LrQFLDGD6D~GHGD Universidade Federal de Santa Maria – Porto Alegre, RS Resumo Objetivo: Descrever observações morfológicas sobre o septo interatrial em fetos normais, especialmente o forame oval e o septo primeiro, de forma a comparar a excursão do septo primeiro com o diâmetro do forame oval. Métodos: As medidas da excursão do septo primeiro (ESP) em direção ao átrio esquerdo (AE) e do diâmetro do forame oval (DFO) foram realizadas em corações de dez fetos humanos formolizados com 28 a 36 semanas. Os cortes histológicos foram feitos no FO, SP, septo segundo e nos AE e AD. Resultados: Os resultados da análise anatômica estão expressos em amplitude das medidas do DFO e da ESP: 3 fetos com idade gestacional (IG) presumida de 28 semanas, DFO (3,1-3,5 mm) e ESP (2,8-3,1 mm); 4 fetos com IG presumida de 34 semanas, DFO (3,3-3,5 mm) e ESP (4,0-5,0 mm); e 3 fetos com IG presumida de 36 semanas, DFO (3,3-4,5 mm) e ESP (6,0-9,0). Foram identificadas fibras musculares cardíacas no SP e no segundo. Conclusão: Pode-se sugerir que o SP apresenta caráter ativo devido às fibras musculares que o constituem, influenciando o fluxo sangüíneo através do FO, a mobilidade do SP e a sua excursão para o interior do AE. -

Embryology-1.Pdf
EMBRYOLOGY COURSE CONTENT COMPETENCIES The first year medical student should be able to understand and explain the principles of fertilization, contraception, stages of early development of the embryo, development of various organ systems; developmental basis of congenital defects, twinning and teratology. GENERAL EMBRYOLOGY INTRODUCTION Stages of human life Prenatal – Zygote, pre-embryonic, embryonic, foetal, birth events Postnatal – Neonatal, infancy, childhood, prepubertal, pubertal, adolescent, adult - young, middle age, old age, death events Ontogeny, trimester, viability, abortion, miscarriage, medical termination of pregnancy, conceptus, abortus Terms of reference — Cranial, rostral, caudal, dorsal, ventral, lateral, medial, median, planes of section Level 3: Ontogeny in relation to phylogeny – The law of recapitulation; “Critical period”; Congenital vs. hereditary malformations; Investigations - USG, amniocentesis, chorionic villus biopsy, fetoscopy, teratology and its significance with respect to obstetrics, paediatrics; Intrauterine surgery; History of embryology GAMETOGENESIS AND FERTILISATION Menstrual cycle with reference to other reproductive cycles, concept of “first day of last menstrual period”, germ cell transport and fertilisation, sperm capacitation, acrosome reaction, zona reaction, methods of contraception, sex determination Level 2: Reference to genetics, abnormal gametogenesis, abnormal germ cells – morphology, abnormal chromosomal contents, biological significance, conception, assisted reproductive techniques -

Atrioventricular Node (AVN) Originates from (I) the Left Wall of Sinus Venosus and (Ii) Superior Endocardial Cushion of the Atriventricular Canal
W.S. O School of Biomedical Sciences, University of Hong Kong Objectives: • Describe early angiogenesis. • Describe the heart tube formation. • Describe the partitioning into a 4- chambered heart. • List the formation of heart valves and the conducting system in the heart. • Explain the basis of congenital heart malformation. Day 25 Day 20 Heart tubes and Intraembryonic embryonic vessels circulation established develop 6 weeks 4-5 weeks Heart tube partitions into 4 Aortic arches heart chambers transform 8 weeks Heart valves Birth completed Foramen ovale closes with increased blood flow from lungs Blood and blood vessels Primitive blood cells Angioblast (mesodermal) Endothelial cells Connective tissue surrounding mesoderm Smooth muscles Blood vessels formation The three parts of circulation: vitelline, chorionic & intraembryonic Molecular regulation of cardiac development • Heart induction – BMPs secreted by endoderm and lateral plate mesoderm together with inhibition of WNT in the anterior part of the embryo induce the expression of NKX-2.5 in the heart -forming region . • Cardiac looping - is induced by the nodal and lefty2 genes. These two genes induce the transcription factor of PITX2 in deposition of extracellular matrix in looping. Development of the heart - 1 1. Cardiogenic plate appears around 3rd week Anterior to prochordal plate Pericardial coelom develop around heart tube 2. Rotation of heart primordia –180 rotation 3. Fusion of heart tubes around 4th week; 4. Differentiation of the heart tube tissue Epicardium – connective tissue Myocardium – cardiac muscle Subendocardium – spongy reticular tissue Endocardium - endothelium Embryonic development of the heart. (a) Endocardial heart tubes start to fuse, (b) Fusion of heart tubes complete, (c) Division of heart tube into dilated segments, (d) Looping of the heart tube, (e) Looping complete, (f) Frontal section of heart tube. -

Anatomy of the True Interatrial Septum for Transseptal Access to the Left Atrium
Annals of Anatomy 205 (2016) 60–64 Contents lists available at ScienceDirect Annals of Anatomy jou rnal homepage: www.elsevier.com/locate/aanat Research article Anatomy of the true interatrial septum for transseptal access to the left atrium 1 ∗,1 Wiesława Klimek-Piotrowska , Mateusz K. Hołda , Mateusz Koziej, Katarzyna Piatek,˛ Jakub Hołda Department of Anatomy, Jagiellonian University Medical College, Cracow, Poland a r t i c l e i n f o a b s t r a c t Article history: Clinical anatomy of the interatrial septum is treacherous, difficult and its unfamiliarity can cause many Received 6 January 2016 serious complications. This work aims to create an anatomical map of the true interatrial septum. An Received in revised form 23 January 2016 appreciation of the anatomical situation is essential for safe and efficacious transseptal access from the Accepted 25 January 2016 right atrium to the left heart chambers. Examination of 135 autopsied human hearts (Caucasian) of 2 both sexes (28% females) aged from 19 to 94 years old (47.0 ± 18.2) with BMI = 27.1 ± 6.0 kg/m was Keywords: conducted. Focus was specifically targeted on the assessment of the fossa ovalis, patent foramen ovale Transseptal puncture (PFO), and right-sided septal pouch (RSP) morphology. Mean values of cranio-caudal and antero-posterior Septal pouch fossa ovalis diameters were 12.1 ± 3.6 and 14.1 ± 3.6 mm, respectively. The fossa ovalis was situated an Patent foramen ovale average of 10.1 ± 4.4 mm above the inferior vena cava ostium, 20.7 ± 5.2 mm from the right atrioventric- Fossa ovalis ular ring, and 12.6 ± 5.2 mm under the right atrium roof. -

Cardiovascular System - Accessscience from Mcgraw-Hill Education
Cardiovascular system - AccessScience from McGraw-Hill Education http://accessscience.com/content/109900 (http://accessscience.com/) Article by: Weichert, Charles K. College of Arts and Sciences, University of Cincinnati, Cincinnati, Ohio. Copenhaver, W. M. College of Physicians and Surgeons, Columbia University, New York; Department of Biological Structures, School of Medicine, University of Miami, Miami, Florida. Ebert, James D. Department of Embryology, Carnegie Institution, Washington, DC. Patten, Bradley M. Department of Anatomy, University of Michigan, Ann Arbor, Michigan. Jones, David R. Department of Zoology, University of British Columbia, Vancouver, Canada. Publication year: 2014 DOI: http://dx.doi.org/10.1036/1097-8542.109900 (http://dx.doi.org/10.1036/1097-8542.109900) Content Comparative Anatomy Embryogenesis of blood vessels Balancing ventricular output Heart Angiogenesis Human Postnatal Circulation Arterial system Circulatory system morphogenesis Pulmonary circuit and ductus Venous system Primitive venous system Physiological aspects of transition Comparative Embryology Functional Development of Heart Comparative Physiology Heart Contractions of the heart General physiology of circulation Tubular heart formation Heart-forming areas Microcirculation Cardiac loop and regional development Contractile proteins Heart Formation of definitive heart Synthesis of contractile proteins Arteries Partitioning of mammalian heart Action of inhibitors Venous system Division of atrium and ventricles Human Fetal Circulation at Term Bibliography