A Snapshot of Transdermal and Topical Drug Delivery Research in Canada
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
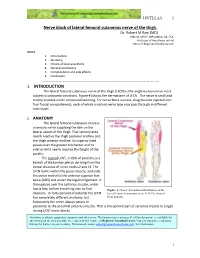
Nerve Block of Lateral Femoral Cutaneous Nerve of the Thigh
18VTLLAA 1 Nerve block of lateral femoral cutaneous nerve of the thigh. Dr. Robert M Raw (MD) . MBChB, MFGP, MPraxMed, DA, FCA. Professor of Anesthesia retired Editor of Regional-Anesthesia.Com INDEX. 1. Introduction 2. Anatomy 3. Choice of local anesthetic 4. General indications 5. Complications and side effects 6. Conclusion ------------------------------------------------------------------------------------ 1. INTRODUCTION The lateral femoral cutaneous nerve of the thigh (LFCN) is the single human nerve most subject to anatomic variations. Figure #1shows the dermatome of LFCN. The nerve is small and mostly invisible under ultrasound scanning. For nerve block success, drug must be injected into four fascial compartments, each of which a variant nerve type may pass through in different individuals. 2. ANATOMY The lateral femoral cutaneous nerve is a sensory nerve supplying the skin on the lateral aspect of the thigh. That sensory area nearly reaches the thigh posterior midline and the thigh anterior midline. Its superior limit passes over the greater trochanter and its inferior limit nearly reaches the height of the patella. The typical LCNT, in 60% of patients, is a branch of the lumbar plexus deriving from the dorsal divisions of nerve roots L2 and L3. The LFCN forms within the psoas muscle, and exits the pelvis medial to the anterior superior iliac spine (ASIS) and under the inguinal ligament. It then passes over the sartorius muscle, under fascia lata, before branching into its final Figure 1. Classic dermatomal distribution of the divisions. In forty percent of patients the LFCN lateral femoral cutaneous nerve (LFCN), derived has completely different anatomy, but from Sobotta. fortunately the nerve always passes in proximity to the proximal sartorius muscle. -

Transdermal Absorption Preparation
Europäisches Patentamt *EP001522316A1* (19) European Patent Office Office européen des brevets (11) EP 1 522 316 A1 (12) EUROPEAN PATENT APPLICATION published in accordance with Art. 158(3) EPC (43) Date of publication: (51) Int Cl.7: A61K 47/34, A61K 47/10, 13.04.2005 Bulletin 2005/15 A61K 47/14, A61K 9/06, A61K 9/08, A61K 9/12, (21) Application number: 03764126.3 A61K 9/70 (22) Date of filing: 02.07.2003 (86) International application number: PCT/JP2003/008400 (87) International publication number: WO 2004/006960 (22.01.2004 Gazette 2004/04) (84) Designated Contracting States: • OMICHI, Katsuhiro AT BE BG CH CY CZ DE DK EE ES FI FR GB GR Saitama-shi, Saitama 338-0832 (JP) HU IE IT LI LU MC NL PT RO SE SI SK TR • OKADA, Minoru Designated Extension States: Inzai-shi, Chiba 270-1323 (JP) AL LT LV MK • KURAZUMI, Toshiaki Narita-shi, Chiba 286-0011 (JP) (30) Priority: 16.07.2002 JP 2002206565 (74) Representative: Hartz, Nikolai F., Dr. (71) Applicant: SSP Co., Ltd. Wächtershäuser & Hartz Chuo-ku, Tokyo 103-8481 (JP) Patentanwälte Weinstrasse 8 (72) Inventors: 80333 München (DE) • NARUI, Takashi Sakura-shi, Chiba 285-0817 (JP) (54) TRANSDERMAL ABSORPTION PREPARATION (57) A transdermal absorption promotion composi- and transdermal absorption preparation not only exhibit tion comprising the following components (a), (b), and an excellent transdermal absorption promotion effect, (c) and a transdermal absorption preparation compris- but also exhibit superior skin-permeability, even if a drug ing the following components (a), (b), (c), and (d) are having a relatively high lipophilic property and poor disclosed. -

Preparatory: 1 Venous Access and Medication Administration: 4
Preparatory: 1 Venous Access and Medication Administration: 4 W4444444444444444444444444444444444444444444444444444444444444444444444444444444444444444444444444444444444444 UNIT TERMINAL OBJECTIVE 1-4 At the completion of this unit, the EMT-Critical Care Technician student will be able to safely and precisely access the venous circulation and administer medications. COGNITIVE OBJECTIVES At the completion of this unit, the EMT-Critical Care Technician student will be able to: 1-4.1 Review the specific anatomy and physiology pertinent to medication administration. (C-1) 1-4.2 Review mathematical principles. (C-1) 1-4.3 Review mathematical equivalents. (C-1) 1-4.4 Differentiate temperature readings between the Centigrade and Fahrenheit scales. (C-3) 1-4.5 Discuss formulas as a basis for performing drug calculations. (C-1) 1-4.6 Calculate oral and parenteral drug dosages for all emergency medications administered to adults, infants and children. (C-2) 1-4.7 Calculate intravenous infusion rates for adults, infants, and children. (C-2) 1-4.8 Discuss legal aspects affecting medication administration. (C-1) 1-4.9 Discuss the "six rights" of drug administration and correlate these with the principles of medication administration. (C-1) 1-4.10 Discuss medical asepsis and the differences between clean and sterile techniques. (C-1) 1-4.11 Describe use of antiseptics and disinfectants. (C-1) 1-4.12 Describe the use of universal precautions and body substance isolation (BSI) procedures when administering a medication. (C-1) 1-4.13 Describe the indications, equipment needed, techniques utilized, precautions, and general principles of peripheral venous cannulation (Including saline locks). (C-1) 1-4.14 Describe the indications, equipment needed, techniques utilized, precautions, and general principles of intraosseous needle placement and infusion. -

Handbook ESRA
TECHNIQUES HEAD & NECK 4 Intracranial surgery p. 3 Eye blocks p. 5 Face anatomy p. 16 Face particularity p. 23 Ophtalmic nerve blocks p. 27 Maxillary nerve blocks p. 33 Mandibular nerve blocks p. 46 THORAX & ABDOMEN 50 Epidural anaesthesia in Cardio-thoracic surgery p. 50 Ilioinguinal-Iliohypogastric block p. 55 Peri-umbilical & Rectus sheath block p. 57 Pudendal block p. 58 UPPER LIMB 61 Choice of a technique p. 61 Brachial plexus anatomy p. 65 Interscalen block p. 68 Supraclavicular blocks p. 73 Infraclavicular blocks p. 80 Axillary block p. 83 LOWER LIMB 90 Lumbar plexus block p. 90 Iliofascial block p. 100 Obturator block p. 102 Sciatic blocks o Sciatic blocks - parasacral nerve approach p. 109 o Sciatic blocks - posterior popliteal approach p. 115 Ankle blocks p. 119 AXIAL BLOCKS 123 Lumbar epidural p. 123 OBSTETRICS AXIAL BLOCKS 126 Epidural p. 126 PERIPHERAL BLOCKS Pudendal block p. 58 2 Aknowledgement The provenience of the materials included in this handbook is from the Learning Zone on the official site of “European Society of Regional Anesthesia and Pain Therapy”. http://www.esra-learning.com/ 2007 3 HEAD & TABLE OF CONTENTS NECK • Intracranial surgery • Eye blocks • Face anatomy • Face particularity • Ophtalmic nerve blocks • Maxillary nerve blocks • Mandibular nerve blocks • Cervical plexus blocks HEAD & INTRACRANIAL SURGERY NECK Paul J. Zetlaoui, M.D. Kremlin-Bicetre - France In intra-cranial neurosurgery, scalp infiltration aims to prevent systematic and cerebral hemodynamic variations, contemporary of skin incision. The potential morbidity of these hypertension-tachycardia episodes, even in patients profoundly anaesthetized, is secondary in the increase of the cerebral blood flow and in its deleterious consequences on intra-cranial pressure in these compromised patients. -

The Development of an Intramuscular Injection Simulation for Nursing Students
Open Access Technical Report DOI: 10.7759/cureus.12366 The Development of an Intramuscular Injection Simulation for Nursing Students Julia Micallef 1 , Artur Arutiunian 1 , Adam Dubrowski 1 1. Health Sciences, Ontario Tech University, Oshawa, CAN Corresponding author: Adam Dubrowski, [email protected] Abstract Intramuscular (IM) injections are preferred over subcutaneous injections for administering medicine such as epinephrine and vaccines as the muscle tissue contains an increased vascular supply that provides ideal absorption of the drug being administered. However, administering an IM injection requires clinical judgment when choosing the injection site, understanding the relevant anatomy and physiology as well as the principles and techniques for administering an IM injection. Therefore, it is essential to learn and perform IM injections using injection simulators to practice the skill before administering to a real patient. Current IM injection simulators either favor realism at the expense of standardization or are expensive but do not provide a realistic experience. Therefore, it is imperative to develop an inexpensive but realistic intramuscular injection simulator that can be used to train nursing students so that they can be prepared for when they enter the clinical setting. This technical report aims to provide an overview of the development of an inexpensive and realistic deltoid simulator geared to teach nursing students the skill of IM injections. After development, the IM simulators were tested and validated by practicing nurses. An 18-item survey was administered to the nurses, and results indicated positive feedback about the realism of the simulator, in comparison to previous models used, such as the Wallcur® PRACTI-Injecta Pads (Wallcur LLC, San Diego, CA). -

The Digestive System
69 chapter four THE DIGESTIVE SYSTEM THE DIGESTIVE SYSTEM The digestive system is structurally divided into two main parts: a long, winding tube that carries food through its length, and a series of supportive organs outside of the tube. The long tube is called the gastrointestinal (GI) tract. The GI tract extends from the mouth to the anus, and consists of the mouth, or oral cavity, the pharynx, the esophagus, the stomach, the small intestine, and the large intes- tine. It is here that the functions of mechanical digestion, chemical digestion, absorption of nutrients and water, and release of solid waste material take place. The supportive organs that lie outside the GI tract are known as accessory organs, and include the teeth, salivary glands, liver, gallbladder, and pancreas. Because most organs of the digestive system lie within body cavities, you will perform a dissection procedure that exposes the cavities before you begin identifying individual organs. You will also observe the cavities and their associated membranes before proceeding with your study of the digestive system. EXPOSING THE BODY CAVITIES should feel like the wall of a stretched balloon. With your skinned cat on its dorsal side, examine the cutting lines shown in Figure 4.1 and plan 2. Extend the cut laterally in both direc- out your dissection. Note that the numbers tions, roughly 4 inches, still working with indicate the sequence of the cutting procedure. your scissors. Cut in a curved pattern as Palpate the long, bony sternum and the softer, shown in Figure 4.1, which follows the cartilaginous xiphoid process to find the ventral contour of the diaphragm. -

The Role of Deformable Liposome Characteristics on Skin Permeability of Meloxicam: Optimal Transfersome As Transdermal Delivery Carriers
Send Orders for Reprints to [email protected] The Open Conference Proceedings Journal, 2013, 4, 87-92 87 Open Access The Role of Deformable Liposome Characteristics on Skin Permeability of Meloxicam: Optimal Transfersome as Transdermal Delivery Carriers Sureewan Duangjit1,2, Praneet Opanasopit1, Theerasak Rojarata1, Yasuko Obata2, Yoshinori Oniki2, Kozo Takayama2 and Tanasait Ngawhirunpat1,* 1Department of Pharmaceutical Technology, Faculty of Pharmacy, Silpakorn University, Nakhon Pathom 73000, Thailand 2Department of Pharmaceutics, Hoshi University, Ebara 2-4-41, Shinagawa-ku, Tokyo 142-8501, Japan Abstract: The role of deformable liposomes characteristics on skin permeability has evoked considerable interest, since the articles reporting the effectiveness of transfersomes for skin delivery were increasingly published. Several reports focus on the effect of formulation factor which directly affected the transfersome’s skin permeability. However, the effect of formulation factors was not fully understood as the contradictory results. To clarify this problem, the reliable statistical techniques, excellent experimental design and systematical variation were used in this study. Transfersomes loaded meloxicam containing controlled amount of phosphatidylcholine (PC), cholesterol (Chol), type of surfactant (hydrophilic part, lipophilic part ) were prepared and investigated for the physicochemical characteristics (e.g., size, size distribution, charge, elasticity, drug content, morphology) and skin permeability. The results indicated -

Pulmonary Delivery of Biological Drugs
pharmaceutics Review Pulmonary Delivery of Biological Drugs Wanling Liang 1,*, Harry W. Pan 1 , Driton Vllasaliu 2 and Jenny K. W. Lam 1 1 Department of Pharmacology and Pharmacy, Li Ka Shing Faculty of Medicine, The University of Hong Kong, 21 Sassoon Road, Pokfulam, Hong Kong, China; [email protected] (H.W.P.); [email protected] (J.K.W.L.) 2 School of Cancer and Pharmaceutical Sciences, King’s College London, 150 Stamford Street, London SE1 9NH, UK; [email protected] * Correspondence: [email protected]; Tel.: +852-3917-9024 Received: 15 September 2020; Accepted: 20 October 2020; Published: 26 October 2020 Abstract: In the last decade, biological drugs have rapidly proliferated and have now become an important therapeutic modality. This is because of their high potency, high specificity and desirable safety profile. The majority of biological drugs are peptide- and protein-based therapeutics with poor oral bioavailability. They are normally administered by parenteral injection (with a very few exceptions). Pulmonary delivery is an attractive non-invasive alternative route of administration for local and systemic delivery of biologics with immense potential to treat various diseases, including diabetes, cystic fibrosis, respiratory viral infection and asthma, etc. The massive surface area and extensive vascularisation in the lungs enable rapid absorption and fast onset of action. Despite the benefits of pulmonary delivery, development of inhalable biological drug is a challenging task. There are various anatomical, physiological and immunological barriers that affect the therapeutic efficacy of inhaled formulations. This review assesses the characteristics of biological drugs and the barriers to pulmonary drug delivery. -

Pressure Ulcer Staging Guide
Pressure Ulcer Staging Guide Pressure Ulcer Staging Guide STAGE I STAGE IV Intact skin with non-blanchable Full thickness tissue loss with exposed redness of a localized area usually Reddened area bone, tendon, or muscle. Slough or eschar may be present on some parts Epidermis over a bony prominence. Darkly Epidermis pigmented skin may not have of the wound bed. Often includes undermining and tunneling. The depth visible blanching; its color may Dermis of a stage IV pressure ulcer varies by Dermis differ from the surrounding area. anatomical location. The bridge of the This area may be painful, firm, soft, nose, ear, occiput, and malleolus do not warmer, or cooler as compared to have subcutaneous tissue and these adjacent tissue. Stage I may be Adipose tissue ulcers can be shallow. Stage IV ulcers Adipose tissue difficult to detect in individuals with can extend into muscle and/or Muscle dark skin tones. May indicate "at supporting structures (e.g., fascia, Muscle risk" persons (a heralding sign of Bone tendon, or joint capsule) making risk). osteomyelitis possible. Exposed bone/ Bone tendon is visible or directly palpable. STAGE II DEEP TISSUE INJURY Partial thickness loss of dermis Blister Purple or maroon localized area of Reddened area presenting as a shallow open ulcer discolored intact skin or blood-filled Epidermis with a red pink wound bed, without Epidermis blister due to damage of underlying soft slough. May also present as an tissue from pressure and/or shear. The intact or open/ruptured serum-filled Dermis area may be preceded by tissue that is Dermis blister. -

Bio-Implant Reference Manual
Bio-Implant Reference Manual International Use Only Bio-Implant Reference Manual Saving Lives, Restoring Health is our business. Nowhere is the reality of death more evident than in the decision-making process surrounding tissue and organ donation. It’s a course of action that involves everything from the simple to the complex, from the sadly certain to the certainly optimistic. LifeNet Health takes this tragedy and turns it into hope. Our full line of allograft bio-implants maximizes the precious gift of donated tissue and provides surgeons with the tools they need to improve the lives of patients. By making the finest quality allograft bio-implants easily accessible, we continue to provide exemplary service to clinicians and hospitals. Every year, LifeNet Health distributes over 400,000 bio-implants to meet the urgent needs of hospitals and patients around the world. Our record of safety is unmatched. And our philosophy is simple: When partnering with a bio-implant supplier, your decision should not be based solely on fee, but rather on the overall value you and your patients expect and deserve. At LifeNet Health, we deliver that value by excelling in these critical areas – safety, quality, innovation, service, clinical effectiveness, supply chain reliability and and experience. With LifeNet Health as your primary bio-implant supplier, you are investing in the best possible value to ensure the well-being of your patients and the reputation of your hospital. This is the value of working with LifeNet Health. 2 757-464-4761 x 2000 (OUS) • 888-847-7831 (US & Canada) • ©2014 LifeNet Health, Virginia Beach, VA. -

Avoid Empiric Treatment for Vulvar Skin Disorders
December 1, 2008 • www.familypracticenews.com Women’s Health 27 Avoid Empiric Treatment for Vulvar Skin Disorders BY NANCY WALSH vulva and anus. Hypopigmentation also is characteristic, ment once daily after soaking. “I believe the Temovate New York Bureau with scarring and architectural changes including phimo- brand is much better than the generic, probably because sis of the clitoris, resorption of the labia minora, and nar- of the vehicle,” he said. The corticosteroid should be con- L AKE B UENA V ISTA, FLA. — Empiric treatment rowing of the introitus causing recurrent tearing. It prob- tinued until active disease has resolved, not just for the 2 with corticosteroids should be avoided in patients who pre- ably is autoimmune, because patients have a high incidence weeks specified in the package insert. sent with vulvar symptoms such as burning, itching, pain, of other autoimmune diseases, especially thyroid disease. A second vulvar condition, lichen simplex chronicus, and dyspareunia, according to Dr. Andrew T. Goldstein. Lichen sclerosus can develop at any age, including is characterized by thick, lichenified skin of the labia ma- These patients should have a careful examination of the childhood, and is more common than generally appreci- jora and interlabial sulcus, accompanied by erosions, fis- vulva using a colposcope, and if a lesion is present, a 4- ated, with a prevalence of 1 in 70 women. But “you have suring, and tears in the skin that result from the patient’s mm punch biopsy is warranted. to look for it. The vulva is not just something to separate scratching in her sleep, said Dr. -

Unit 4: Medication Administration Fundamental of Nursing
Unit 4: Medication Administration Fundamental of Nursing Unit 4: Medication Administration: Medication: Is a substance administered for the diagnosis, cure, treatment, relief, or prevention of disease. Six Rights of Medication Administration After paramedics have received the medication or fluid order, they should then administer the drug in question. In performing drug administration, pre-hospital care providers adhere to the six rights of medication administration: 1. Right patient 2. Right medication 3. Right dose 4. Right route 5. Right time 6. Right documentation Basic principle of nurse on drugs administration 1. The nurse must know the drug's prescribed dose, method of administration, actions, expected therapeutic effect, possible interactions with other drugs, and adverse effects. 2. The nurse must know the institution's administration procedures for the client's welfare and the nurse's legal protection. 3. The nurse must Review physician's order for completeness the client's name, date of the order, name of the drug, dose, rout, time of administration, and the physician's signature. 1 Unit 4: Medication Administration Fundamental of Nursing 4. The nurse discusses the medication and its actions with the client; recheck the medication order if the client disagrees with the dose or the physician's order. 5. The nurse must check the physician's order against the client's medication administration record for accuracy. 6. The nurse gives the patient the right to know about the medication he is receiving and the right to refuse it. Routes of Administration A: Enteral Tract Routes The common enteral routes of administration used in general medical practice are as follows: 1.