Hypomagnesemia in Critically Ill Patients Bent-Are Hansen1 and Øyvind Bruserud2*
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Hypokalaemia in a Woman with Eating Disorder
Grand Rounds Vol 11 pages 53–55 Specialities: Acute Medicine; Nephrology; Psychiatry Article Type: Case Report DOI: 10.1102/1470-5206.2011.0013 ß 2011 e-MED Ltd Hypokalaemia in a woman with eating disorder Zachary Z. Brenera, Boris Medvedovskya, James F. Winchestera and Michael Bergmanb aDivision of Nephrology, Department of Medicine, Beth Israel Medical Center, Albert Einstein School of Medicine of Yeshiva University, New York, USA; bDepartment of Medicine, Campus Golda, Rabin Medical Center, Petah-Tikva, Tel-Aviv University, Israel Corresponding address: Dr Zachary Z. Brener, 350 E. 17th St., Division of Nephrology, Beth Israel Medical Center, New York, NY 10003, USA. Email: [email protected] Date accepted for publication 13 April 2011 Abstract Chronic hypokalaemia often remains a diagnostic challenge, especially in young women without hypertension. A concealed diuretic abuse should be suspected, especially in young women with eating disorders. This case describes a woman with chronic hypokalaemia in whom a thorough medical history and proper laboratory tests were essential to early and accurate diagnosis. Keywords Hypokalaemia; eating disorders; diuretics. Introduction Chronic hypokalaemia often remains a diagnostic challenge, especially in young women without hypertension. After the exclusion of the most obvious causes, a concealed diuretic abuse associated with or without surreptitious vomiting and laxative abuse should be suspected, especially in young women concerned with their body image. A conclusive diagnosis may be difficult as such patients often vigorously deny diuretic intake[1]. Also, only a minority of patients with eating disorders (approximately 6%) abuse diuretics[2–4]. This case describes a woman with chronic hypokalaemia in whom a thorough medical history and proper laboratory tests were essential to an early and accurate diagnosis. -

Vitamin and Mineral Requirements in Human Nutrition
P000i-00xx 3/12/05 8:54 PM Page i Vitamin and mineral requirements in human nutrition Second edition VITPR 3/12/05 16:50 Page ii WHO Library Cataloguing-in-Publication Data Joint FAO/WHO Expert Consultation on Human Vitamin and Mineral Requirements (1998 : Bangkok, Thailand). Vitamin and mineral requirements in human nutrition : report of a joint FAO/WHO expert consultation, Bangkok, Thailand, 21–30 September 1998. 1.Vitamins — standards 2.Micronutrients — standards 3.Trace elements — standards 4.Deficiency diseases — diet therapy 5.Nutritional requirements I.Title. ISBN 92 4 154612 3 (LC/NLM Classification: QU 145) © World Health Organization and Food and Agriculture Organization of the United Nations 2004 All rights reserved. Publications of the World Health Organization can be obtained from Market- ing and Dissemination, World Health Organization, 20 Avenue Appia, 1211 Geneva 27, Switzerland (tel: +41 22 791 2476; fax: +41 22 791 4857; e-mail: [email protected]). Requests for permis- sion to reproduce or translate WHO publications — whether for sale or for noncommercial distri- bution — should be addressed to Publications, at the above address (fax: +41 22 791 4806; e-mail: [email protected]), or to Chief, Publishing and Multimedia Service, Information Division, Food and Agriculture Organization of the United Nations, 00100 Rome, Italy. The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the World Health Organization and the Food and Agriculture Organization of the United Nations concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. -

341 Nutrient Deficiency Or Disease Definition/Cut-Off Value
10/2019 341 Nutrient Deficiency or Disease Definition/Cut-off Value Any currently treated or untreated nutrient deficiency or disease. These include, but are not limited to, Protein Energy Malnutrition, Scurvy, Rickets, Beriberi, Hypocalcemia, Osteomalacia, Vitamin K Deficiency, Pellagra, Xerophthalmia, and Iron Deficiency. Presence of condition diagnosed, documented, or reported by a physician or someone working under a physician’s orders, or as self-reported by applicant/participant/caregiver. See Clarification for more information about self-reporting a diagnosis. Participant Category and Priority Level Category Priority Pregnant Women 1 Breastfeeding Women 1 Non-Breastfeeding Women 6 Infants 1 Children 3 Justification Nutrient deficiencies or diseases can be the result of poor nutritional intake, chronic health conditions, acute health conditions, medications, altered nutrient metabolism, or a combination of these factors, and can impact the levels of both macronutrients and micronutrients in the body. They can lead to alterations in energy metabolism, immune function, cognitive function, bone formation, and/or muscle function, as well as growth and development if the deficiency is present during fetal development and early childhood. The Centers for Disease Control and Prevention (CDC) estimates that less than 10% of the United States population has nutrient deficiencies; however, nutrient deficiencies vary by age, gender, and/or race and ethnicity (1). For certain segments of the population, nutrient deficiencies may be as high as one third of the population (1). Intake patterns of individuals can lead to nutrient inadequacy or nutrient deficiencies among the general population. Intakes of nutrients that are routinely below the Dietary Reference Intakes (DRI) can lead to a decrease in how much of the nutrient is stored in the body and how much is available for biological functions. -

Magnesium Deficiency: a Possible Cause of Thiamine Refractoriness in Wernicke-Korsakoff Encephalopathy
J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp.37.8.959 on 1 August 1974. Downloaded from Journal of Neurology, Neurosurgery, and Psychiatry, 1974, 37, 959-962 Magnesium deficiency: a possible cause of thiamine refractoriness in Wernicke-Korsakoff encephalopathy D. C. TRAVIESA From the Department ofNeurology, University ofMiami School of Medicine, Miami, Florida, U.S.A. SYNOPSIS The determination of blood transketolase before and serially after thiamine administra- tion, and the response of clinical symptomatology after thiamine are reported in two normo- magnesaemic patients and one hypomagnesaemic patient with acute Wernicke-Korsakoff encephalopathy. The response of the depressed blood transketolase and the clinical symptoms was retarded in the hypomagnesaemic patient. Correction of hypomagnesaemia was accompanied by the recovery of blood transketolase activity and total clearing of the ophthalmoplegia in this patient, guest. Protected by copyright. suggesting that hypomagnesaemia may be a cause of the occasional thiamine refractoriness of these patients. Previous studies have shown that the clinical vestibular nuclei (Prickett, 1934; Dreyfus and entity of Wernicke-Korsakoff encephalopathy is Victor, 1961; Dreyfus, 1965). related to an exclusive deficiency of thiamine (Phillips et al., 1952). Furthermore, improvement Blood transketolase activity is markedly re- in clinical symptoms, once the syndrome has de- duced in patients with Wernicke-Korsakoff veloped, occurs only with the repletion of thi- encephalopathy and serial assays in these patients amine. It is this obvious and seemingly pure usually reveal a rapid and essentially complete causal relationship of thiamine deficiency and recovery of the reduced blood transketolase Wernicke-Korsakoff encephalopathy that neces- activity after the parental administration of sitates the total understanding of thiamine thiamine (Brin, 1962; Dreyfus, 1962). -

Magnesium: the Forgotten Electrolyte—A Review on Hypomagnesemia
medical sciences Review Magnesium: The Forgotten Electrolyte—A Review on Hypomagnesemia Faheemuddin Ahmed 1,* and Abdul Mohammed 2 1 OSF Saint Anthony Medical Center, 5666 E State St, Rockford, IL 61108, USA 2 Advocate Illinois Masonic Medical Center, 833 W Wellington Ave, Chicago, IL 60657, USA; [email protected] * Correspondence: [email protected] Received: 20 February 2019; Accepted: 2 April 2019; Published: 4 April 2019 Abstract: Magnesium is the fourth most abundant cation in the body and the second most abundant intracellular cation. It plays an important role in different organ systems at the cellular and enzymatic levels. Despite its importance, it still has not received the needed attention either in the medical literature or in clinical practice in comparison to other electrolytes like sodium, potassium, and calcium. Hypomagnesemia can lead to many clinical manifestations with some being life-threatening. The reported incidence is less likely than expected in the general population. We present a comprehensive review of different aspects of magnesium physiology and hypomagnesemia which can help clinicians in understanding, identifying, and treating this disorder. Keywords: magnesium; proton pump inhibitors; diuretics; hypomagnesemia 1. Introduction Magnesium is one of the most abundant cation in the body as well as an abundant intracellular cation. It plays an important role in molecular, biochemical, physiological, and pharmacological functions in the body. The importance of magnesium is well known, but still it is the forgotten electrolyte. The reason for it not getting the needed attention is because of rare symptomatology until levels are really low and also because of a lack of proper understanding of magnesium physiology. -
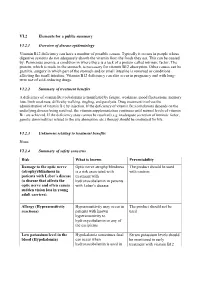
VI.2 Elements for a Public Summary VI.2.1 Overview of Disease Epidemiology Vitamin B12 Deficiency Can Have a Number of Possible
VI.2 Elements for a public summary VI.2.1 Overview of disease epidemiology Vitamin B12 deficiency can have a number of possible causes. Typically it occurs in people whose digestive systems do not adequately absorb the vitamin from the foods they eat. This can be caused by: Pernicious anemia, a condition in where there is a lack of a protein called intrinsic factor. The protein, which is made in the stomach, is necessary for vitamin B12 absorption. Other causes can be gastritis, surgery in which part of the stomach and/or small intestine is removed or conditions affecting the small intestine. Vitamin B12 deficiency can also occur in pregnancy and with long- term use of acid-reducing drugs. VI.2.2 Summary of treatment benefits A deficiency of vitamin B12 (cobalamin) is manifested by fatigue, weakness, mood fluctuations, memory loss, limb weakness, difficulty walking, tingling, and paralysis. Drug treatment involves the administration of vitamin B12 by injection. If the deficiency of vitamin B12 (cobalamin) depends on the underlying disease being resolved, the vitamin supplementation continues until normal levels of vitamin B12 are achieved. If the deficiency state cannot be resolved (e.g. inadequate secretion of intrinsic factor, genetic abnormalities related to the site absorption, etc.) therapy should be continued for life. VI.2.3 Unknowns relating to treatment benefits None. VI.2.4 Summary of safety concerns Risk What is known Preventability Damage to the optic nerve Optic nerve atrophy/blindness The product should be used (atrophy)/blindness in is a risk associated with with caution. patients with Leber´s disease treatment with (a disease that affects the hydroxocobalamin in patients optic nerve and often causes with Leber´s disease. -

Mechanism of Hypokalemia in Magnesium Deficiency
SCIENCE IN RENAL MEDICINE www.jasn.org Mechanism of Hypokalemia in Magnesium Deficiency Chou-Long Huang*† and Elizabeth Kuo* *Department of Medicine, †Charles and Jane Pak Center for Mineral Metabolism and Clinical Research, University of Texas Southwestern Medical Center, Dallas, Texas ABSTRACT deficiency is likely associated with en- ϩ Magnesium deficiency is frequently associated with hypokalemia. Concomitant hanced renal K excretion. To support magnesium deficiency aggravates hypokalemia and renders it refractory to treat- this idea, Baehler et al.5 showed that ad- ment by potassium. Herein is reviewed literature suggesting that magnesium ministration of magnesium decreases ϩ deficiency exacerbates potassium wasting by increasing distal potassium secre- urinary K excretion and increases se- ϩ tion. A decrease in intracellular magnesium, caused by magnesium deficiency, rum K levels in a patient with Bartter releases the magnesium-mediated inhibition of ROMK channels and increases disease with combined hypomagnesemia potassium secretion. Magnesium deficiency alone, however, does not necessarily and hypokalemia. Similarly, magnesium ϩ cause hypokalemia. An increase in distal sodium delivery or elevated aldosterone replacement alone (without K ) in- ϩ levels may be required for exacerbating potassium wasting in magnesium creases serum K levels in individuals deficiency. who have hypokalemia and hypomag- nesemia and receive thiazide treatment.6 J Am Soc Nephrol 18: 2649–2652, 2007. doi: 10.1681/ASN.2007070792 Magnesium administration decreased urinary Kϩ excretion in these individuals (Dr. Charles Pak, personal communica- Hypokalemia is among the most fre- cin B, cisplatin, etc. Concomitant magne- tion, UT Southwestern Medical Center quently encountered fluid and electro- sium deficiency has long been appreci- at Dallas, July 13, 2007). -

An Infant with Chronic Hypernatremia
European Journal of Endocrinology (2006) 155 S141–S144 ISSN 0804-4643 An infant with chronic hypernatremia M L Marcovecchio Department of Paediatrics, University of Chieti, Via dei Vestini 5, 66100 Chieti, Italy (Correspondence should be addressed to M L Marcovecchio; Email: [email protected]) Abstract A 4-month-old boy was presented with failure to thrive, refusal to feed, delayed motor development, truncal hypotonia, and head lag. His plasma osmolality and sodium were significantly high, while his urine osmolality was inappropriately low and did not increase after desmopressin administration. Despite his hyperosmolality, he presented with a lack of thirst and became clearly polyuric and polydipsic only at the age of 2 years. Initial treatment with indomethacin was ineffective, while the combination of hydrochlorothiazide and amiloride was effective and well tolerated. European Journal of Endocrinology 155 S141–S144 Introduction (61 ml/kg) respectively. Other investigations including thyroid and adrenal function were normal. He was Chronic hypernatremia in young patients is generally refusing oral fluids despite being hypernatremic. the result of alterations in the mechanisms controlling A cranial magnetic resonance imaging scan excluded fluid balance (1). Excessive water loss, as in diabetes structural abnormalities in the hypophysis, hypo- insipidus, or an inadequate fluid intake, as in adipsic thalamus and surrounding area. There was no response hypernatremia, may be the underlying cause. The to 0.3 mg 1-desamino-8-D-arginine-vasopressin (DDAVP) differential diagnosis between these conditions is given intravenously (osmolality pre- 374 mosmol/kg; important in order to choose the appropriate treatment. post- 371 mosmol/kg). This suggested a tubular defect However, pitfalls in the diagnosis are often related to an causing nephrogenic diabetes insipidus (NDI). -

Electrolyte and Acid-Base Disorders Triggered by Aminoglycoside Or Colistin Therapy: a Systematic Review
antibiotics Review Electrolyte and Acid-Base Disorders Triggered by Aminoglycoside or Colistin Therapy: A Systematic Review Martin Scoglio 1,* , Gabriel Bronz 1, Pietro O. Rinoldi 1,2, Pietro B. Faré 3,Céline Betti 1,2, Mario G. Bianchetti 1, Giacomo D. Simonetti 1,2, Viola Gennaro 1, Samuele Renzi 4, Sebastiano A. G. Lava 5 and Gregorio P. Milani 2,6,7 1 Faculty of Biomedicine, Università della Svizzera Italiana, 6900 Lugano, Switzerland; [email protected] (G.B.); [email protected] (P.O.R.); [email protected] (C.B.); [email protected] (M.G.B.); [email protected] (G.D.S.); [email protected] (V.G.) 2 Department of Pediatrics, Pediatric Institute of Southern Switzerland, Ospedale San Giovanni, Ente Ospedaliero Cantonale, 6500 Bellinzona, Switzerland; [email protected] 3 Department of Internal Medicine, Ospedale La Carità, Ente Ospedaliero Cantonale, 6600 Locarno, Switzerland; [email protected] 4 Division of Hematology and Oncology, The Hospital for Sick Children, Toronto, ON M5G 1X8, Canada; [email protected] 5 Pediatric Cardiology Unit, Department of Pediatrics, Centre Hospitalier Universitaire Vaudois, and University of Lausanne, 1011 Lausanne, Switzerland; [email protected] 6 Pediatric Unit, Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, 20122 Milan, Italy 7 Department of Clinical Sciences and Community Health, Università degli Studi di Milano, 20122 Milan, Italy * Correspondence: [email protected] Citation: Scoglio, M.; Bronz, G.; Abstract: Aminoglycoside or colistin therapy may alter the renal tubular function without decreasing Rinoldi, P.O.; Faré, P.B.; Betti, C.; the glomerular filtration rate. This association has never been extensively investigated. -

A Retrospective Case Series of Thiamine Deficiency in Non
Journal of Clinical Medicine Article A Retrospective Case Series of Thiamine Deficiency in Non-Alcoholic Hospitalized Veterans: An Important Cause of Delirium and Falling? Elisabeth Mates 1,2,* , Deepti Alluri 3, Tailer Artis 2 and Mark S. Riddle 1,2 1 Medicine Department, Veterans Affairs Sierra Nevada Healthcare System, Reno, NV 89502, USA; [email protected] 2 School of Medicine, University of Nevada, Reno, NV 89502, USA; [email protected] 3 Sound Physicians, Lutheran Hospital, Fort Wayne, IN 46804, USA; [email protected] * Correspondence: [email protected] Abstract: Thiamine deficiency (TD) in non-alcoholic hospitalized patients causes a variety of non- specific symptoms. Studies suggest it is not rare in acutely and chronically ill individuals in high income countries and is underdiagnosed. Our aim is to demonstrate data which help define the risk factors and constellation of symptoms of TD in this population. We describe 36 cases of TD in hospitalized non-alcoholic veterans over 5 years. Clinical and laboratory data were extracted by chart review +/− 4 weeks of plasma thiamine level 7 nmol/L or less. Ninety-seven percent had two or more chronic inflammatory conditions (CICs) and 83% had one or more acute inflammatory conditions (AICs). Of possible etiologies of TD 97% had two or more of: insufficient intake, inflammatory stress, or increased losses. Seventy-five percent experienced 5% or more weight loss. Ninety-two percent had symptoms with the most common being weakness or falling (75%) followed by neuropsychiatric Citation: Mates, E.; Alluri, D.; Artis, manifestations (72%), gastrointestinal dysfunction (53%), and ataxia (42%). We conclude that TD is T.; Riddle, M.S. -

Thiamine and Magnesium Deficiencies
Medical Hypotheses 84 (2015) 129–134 Contents lists available at ScienceDirect Medical Hypotheses journal homepage: www.elsevier.com/locate/mehy Thiamine and magnesium deficiencies: Keys to disease ⇑ D. Lonsdale Associate Emeritus, Cleveland Clinic, Cleveland, OH, United States article info abstract Article history: Thiamine deficiency (TD) is accepted as the cause of beriberi because of its action in the metabolism of Received 21 September 2014 simple carbohydrates, mainly as the rate limiting cofactor for the dehydrogenases of pyruvate and alpha- Accepted 6 December 2014 ketoglutarate, both being critical to the action of the citric acid cycle. Transketolase, dependent on thiamine and magnesium, occurs twice in the oxidative pentose pathway, important in production of reducing equivalents. Thiamine is also a cofactor in the dehydrogenase complex in the degradation of the branched chain amino acids, leucine, isoleucine and valine. In spite of these well accepted facts, the overall clinical effects of TD are still poorly understood. Because of the discovery of 2-hydroxyacyl- CoA lyase (HACL1) as the first peroxisomal enzyme in mammals found to be dependent on thiamine pyrophosphate (TPP) and the ability of thiamine to bind with prion protein, these factors should improve our clinical approach to TD. HACL1 has two important roles in alpha oxidation, the degradation of phy- tanic acid and shortening of 2-hydroxy long-chain fatty acids so that they can be degraded further by beta oxidation. The downstream effects of a lack of efficiency in this enzyme would be expected to be critical in normal brain metabolism. Although TD has been shown experimentally to produce reversible damage to mitochondria and there are many other causes of mitochondrial dysfunction, finding TD as the poten- tial biochemical lesion would help in differential diagnosis. -

The Serum Sodium, Potassium and Calcium Levels in Children 6- 59 Months of Age with Severe Acute Malnutrition
ORIGINAL ARTICLE The Serum Sodium, Potassium and Calcium Levels in Children 6- 59 Months of Age with Severe Acute Malnutrition BILQUEES FATIMA1, MUHAMMAD AMIN SHEIKH2, MALIK MUHAMMAD NAEEM3 ABSTRACT Aim: To study the serum sodium, potassium and calcium levels in cases of SAM and their comparison in cases with and without diarrhea Methods: This cross sectional study was conducted in the pediatric unit II, Bahawal Victoria Hospital, Bahawalpur from January 2016 to June 2016 in children having age between 6 to 59 months admitting with a diagnosis of SAM. The patients were divided into two groups, Group A with diarrhea and group B without diarrhea. Blood was taken for serum sodium, potassium and calcium. Results: 100 patients were included. Their mean±SD age in months was 23.56±3.80. 62% were males. Serum sodium (mean±SD) was 138.46±4.14 mmol/L, potassium 3.961±0.691 mmol/L and calcium 8.359±0.61 mg/dl. 67 cases presented with (Group A) while 33 without diarrhea (Group B). The serum sodium (mean±SD) was 138.51±4.22 mmol/L in group A while 138.36± 4.05 in group B (p 0.1), the potassium 3.89±0.67 in group A while 4.1±0.72 in group B (p 0.009) and calcium (mg/dL) 8.39±0.6 in group A while 8.34 ±0.73 mg/dL in group B (0.71). There was isolated hyponatremia in 10.4% cases in group A and 9.1% in group B (p 0.83) while isolated hypokalemia in 26.9% cases in group A and 18.2% in group B (p 0.34).