Total of 10 Paces Only Ma V Be Xeroxed
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Heritage Impact Assessment Proposed Afrisam Cement Plant, Mine and Associated Infrastructure in Saldanha, Western Cape
HERITAGE IMPACT ASSESSMENT PROPOSED AFRISAM CEMENT PLANT, MINE AND ASSOCIATED INFRASTRUCTURE IN SALDANHA, WESTERN CAPE As part of the Environmental Impact Assessment Prepared for Aurecon South Africa (Pty) Ltd On behalf of Afrisam (South Africa) (Pty) Ltd September 2011 Prepared by: David Halkett ACO Associates cc 8 Jacobs Ladder St James 7945 Phone 0731418606 Email: [email protected] 1 DECLARATION by the independent person who compiled a specialist report or undertook a specialist process I …David John Halkett…………………………………, as the appointed independent specialist hereby declare that I: act/ed as the independent specialist in this application; regard the information contained in this report as it relates to my specialist input /study to be true and correct, and do not have and will not have any financial interest in the undertaking of the activity, other than remuneration for work performed in terms of the NEMA, the Environmental Impact Assessment Regulations, 2010 and any specific environmental management Act; have and will not have no vested interest in the proposed activity proceeding; have disclosed, to the applicant, EAP and competent authority, any material information that have or may have the potential to influence the decision of the competent authority or the objectivity of any report, plan or document required in terms of the NEMA, the Environmental Impact Assessment Regulations, 2010 and any specific environmental management Act; am fully aware of and meet the responsibilities in terms of NEMA, the Environmental Impact Assessment Regulations, 2010 (specifically in terms of regulation 17 of GN No. R. 543) and any specific environmental management Act, and that failure to comply with these requirements may constitute and result in disqualification; have provided the competent authority with access to all information at my disposal regarding the application, whether such information is favourable to the applicant or not; and am aware that a false declaration is an offence in terms of regulation 71 of GN No. -
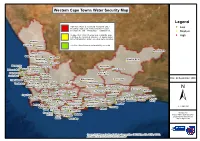
Legend High Risk: Water Demand and Availability Under (! Threat That Requires Urgent Interventions in Resource Low Development and Infrastructure Establishment
Western Cape Towns Water Security Map Legend High Risk: Water Demand and Availability under (! threat that requires urgent interventions in resource Low development and infrastructure establishment. (! Medium Medium Risk: Water Demand and Availability under (! High restriction due to lack of assurance of supply and/or lack of infrastructure and/or exceeding lawful allocation. Lutzville (! Vredendal (! Klawer Low Risk: Water Demand and Availability not at risk (! Murraysburg (! Lamberts Bay (! Graafwater (! (! Wuppertal Clanwilliam (! Beaufort West (! Redelinghuys Citrusdal (! (! Stompneus Eendekuil Merweville Bay Dwarskersbos (! ! (! (! (! Leeu-Gamka Britannia Bay ((!(!Velddrift (! (! Aurora (! Louwville Prince Paternoster (! (! Albert Road (! ! Piketberg Jacobsbaai (! ( (! (! (! (! Hopefield Porterville Saldanha ! Date: 04 September 2020 (! ( Matjies(!fontein Prince Albert Churchhaven Moorreesburg Gouda (! (! ! (! Tulbagh Touwsrivier ( (! Laingsburg Yzerfontein (! R(!iebeek-Wes (!(!Hermon (! (! De Doorns Zoar Darling (! Ceres (! Calitzdorp (! Riebeek-Kasteel Ladismith (! (! ! Dysselsdorp ( (! Oudtshoorn (! Uniondale Paarl (! Worcester Van Wyksdorp (! (! Haarlem (! Montagu (! Volmoed (! Robertson (! (! Cape Town Franschhoek (! Barrydale Karatara (! Ashton (! Ruitersbos ! Plettenberg (! (! (! (! ( Wittedrif Stellenbosch Gena(!dendal Greyton (! Groot Br(!akrivier !Bay(! (!(!(! Suurb(!ra(!ak Heidelberg Riversdale (! (! (! (! (! ( Natures Villiersdorp (! (! B(!randwag Wilderness Knysn(!a (! Bereaville(! (! Albertinia (! Valley ± Grabouw Riviersonderend Slangrivier (! (! (! (! (! Caledon Dana Bay Betty's Bay (! (! (! (! ! Botrivier Klipdale Witsand ( (! (! (! Vlees Bay 1 : 3 000 000 Hawston (! V(!ermo(!nt Napier Malgas Onrus (! Stilbaai He(!rmanus Elim (! Bredasdorp (!(! (! Franskraal Ga(!ns Bay (! Data Source: Arniston Dept of Water and Sanitation Strand Pearly L'Agulhas (!(! Dept of Local Government Beach Struis Bay Dept of Agriculture Source: Esri, Maxar, GeoEye, Earthstar Geographics, CNES/Airbus DS, USDA, USGS, AeroGRID, IGN, and the GIS User Community. -

SLR CV Template
CURRICULUM VITAE ELOISE COSTANDIUS SENIOR ENVIRONMENTAL CONSULTANT Environmental Management, Planning & Approvals, South Africa QUALIFICATIONS Pr.Sci.Nat. 2010 Professional Natural Scientist (Environmental Science) with the South African Council for Natural Scientific Professions MSc 2005 Ecological Assessment BSc (Hons) 2002 Zoology BSc 2001 Biodiversity and Ecology, Botany, Zoology z EXPERTISE Eloise has worked as an environmental assessment practitioner since 2005 and has been involved in a number of projects covering a range of environmental Environmental Impact disciplines, including Basic Assessments, Environmental Impact Assessments, Assessment Environmental Management Programmes, Maintenance Management Plans, Public Participation Environmental Control Officer services and Public Consultation and Facilitation. In Environmental Auditing her 12 years as a consultant, she has gained experience in projects relating to oil Terrestrial Fauna and gas exploration, road infrastructure, renewable energy and housing and Assessment industrial developments. The majority of her work has been based in South Africa, but she also has experience working in Namibia and Mauritius. In addition, she has also undertaken a number of terrestrial fauna assessments as part of EIA specialist teams. PROJECTS Oil and Gas Spectrum Geo Ltd – Proposed EMP process for a Reconnaissance Permit Application to acquire 2D seismic data 2D Speculative Seismic Survey in a large area off the South-West and South coast of South Africa. Eloise was the off the South-West and South project manager and compiled the EMP report, undertook the required public Coast, South Africa (2018) participation process and managed the appointed specialists. Spectrum Geo Ltd - Proposed EMP process for a Reconnaissance Permit Application to acquire 2D seismic data 2D Speculative Seismic Survey within a large offshore area off the South and Southwest coast of South Africa. -

Phase 1 Archaeological Impact Assessment Erf 299 Jacobsbaai Vredenburg-Saldanha Municipality Western Cape Province
PHASE 1 ARCHAEOLOGICAL IMPACT ASSESSMENT ERF 299 JACOBSBAAI VREDENBURG-SALDANHA MUNICIPALITY WESTERN CAPE PROVINCE Prepared for BKS (PTY) LTD By Jonathan Kaplan Agency for Cultural Resource Management P.O. Box 159 Riebeek West 7306 Ph/Fax: 022 461 2755 Cellular: 082 321 0172 E-mail: [email protected] DECEMBER 2004 EXECUTIVE SUMMARY A Phase 1 Archaeological Impact Assessment (AIA) of Erf 299 Jacobsbaai has identified potentially significant impacts to pre-colonial archaeological material that will need to be mitigated prior to development activities. Shovel testing, as a means of determining the significance of the archaeological remains, is recommended. Alternative mitigation measures include creating a permanent shell midden corridor in the northern portion of the affected property in order to protect important archaeological heritage remains. The recommendations are subject to the approval of the Archaeology Palaeontology and Meteorites Committee of Heritage Western Cape, the delegated Provincial Heritage Authority. 2 1. INTRODUCTION 1.1 Background and brief BKS (Pty) Ltd requested that the Agency for Cultural Resource Management undertake a Phase 1 Archaeological Impact Assessment of Erf 299 Jacobsbaai, in Vredenburg- Saldanha Bay, in the Western Cape Province. The proposed rezoning and subdivision of Erf 299 Jacobsbaai is for the purpose of a residential housing development. 132 single residential units are envisaged. A Group Housing component and a small business node is also planned The size of the property is about 41 ha in extent. The aim of the study is to locate, identify and map archaeological sites/remains that may be negatively impacted by the planning and construction of the proposed development, and to propose measures to mitigate against the impact. -

Managing Water As a Constraint to Development with Decision-Support Tools That Promote Integrated Planning: the Case of the Berg Water Management Area
Managing Water as a Constraint to Development with Decision-Support Tools That Promote Integrated Planning: The Case of the Berg Water Management Area C. Pengelly, H. Seyler, N. Fordyce, P. Janse van Vuuren, M. van der Walt, H. van Zyl & J. Kinghorn Report to the Water Research Commission and Western Cape Government by GreenCape Sector Development Agency WRC Report No. TT xxx/xx December 2017 Obtainable from Water Research Commission Private Bag X03 Gezina, 0031 South Africa [email protected] The publication of this report emanates from a project entitled Towards Sustainable Economic Development in Water Constrained Catchments: Tools to Empower Decision Making (WRC Project No. K5/2453) DISCLAIMER This report has been reviewed by the Water Research Commission (WRC) and approved for publication. Approval does not signify that the contents necessarily reflect the views and policies of the WRC, nor does mention of trade names or commercial products constitute endorsement or recommendation for use. ISBN XXXXX Printed in the Republic of South Africa ii Executive summary There is increasing recognition that the combined effects of climate change, population growth and continued urbanisation are exerting pressure on limited water resources. At the same time, economic growth remains vital for alleviating poverty. Therefore, growth is required in spite of significant water resource constraints. At issue then is how to allocate water optimally to enable economic growth, while also ensuring that human needs are met and ecological systems maintained. Understanding of the economic impacts of water access is limited, and there is a lack of tools available to address the trade-offs that may be required when allocating water in a water scarce system. -

Flower Route Map 2017
K o n k i e p en w R31 Lö Narubis Vredeshoop Gawachub R360 Grünau Karasburg Rosh Pinah R360 Ariamsvlei R32 e N14 ng Ora N10 Upington N10 IAi-IAis/Richtersveld Transfrontier Park Augrabies N14 e g Keimoes Kuboes n a Oranjemund r Flower Hotlines O H a ib R359 Holgat Kakamas Alexander Bay Nababeep N14 Nature Reserve R358 Groblershoop N8 N8 Or a For up-to-date information on where to see the Vioolsdrif nge H R27 VIEWING TIPS best owers, please call: Eksteenfontein a r t e b e e Namakwa +27 (0)72 760 6019 N7 i s Pella t Lekkersing t Brak u Weskus +27 (0)63 724 6203 o N10 Pofadder S R383 R383 Aggeneys Flower Hour i R382 Kenhardt To view the owers at their best, choose the hottest Steinkopf R363 Port Nolloth N14 Marydale time of the day, which is from 11h00 to 15h00. It’s the s in extended ower power hour. Respect the ower Tu McDougall’s Bay paradise: Walk with care and don’t trample plants R358 unnecessarily. Please don’t pick any buds, bulbs or N10 specimens, nor disturb any sensitive dune areas. Concordia R361 R355 Nababeep Okiep DISTANCE TABLE Prieska Goegap Nature Reserve Sun Run fels Molyneux Buf R355 Springbok R27 The owers always face the sun. Try and drive towards Nature Reserve Grootmis R355 the sun to enjoy nature’s dazzling display. When viewing Kleinzee Naries i R357 i owers on foot, stand with the sun behind your back. R361 Copperton Certain owers don’t open when it’s overcast. -

Proposed Boulders Wind Farm, West Coast District Municipality, Western Cape Province
PROPOSED BOULDERS WIND FARM, WEST COAST DISTRICT MUNICIPALITY, WESTERN CAPE PROVINCE DEA Reference: 14/12/16/3/3/2/1057 DRAFT ENVIRONMENTAL IMPACT ASSESSMENT REPORT Prepared for: Vredenburg Windfarm (Pty) Ltd. Prepared by: CES EAST LONDON 25 Tecoma Street Berea, East London, 5214 043 726 7809 Also in Grahamstown, Port Elizabeth, Cape Town, Johannesburg and Maputo (Mozambique) www.cesnet.co.za | www.eoh.co.za May 2019 DRAFT Environmental Impact Assessment Report – May 2019 COPYRIGHT INFORMATION This document contains intellectual property and propriety information that is protected by copyright in favour of CES and the specialist consultants. The document may therefore not be reproduced, used or distributed to any third party without the prior written consent of CES. This document is prepared exclusively for submission to Vredenburg Windfarm (Pty) Ltd., and is subject to all confidentiality, copyright and trade secrets, rules intellectual property law and practices of South Africa. CES i Boulders Wind Farm DRAFT Environmental Impact Assessment Report – May 2019 This Report should be cited as follows: CES, May 2019: Proposed Boulders Wind Farm, Environmental Impact Assessment Report, CES East London. REVISIONS TRACKING TABLE CES Report Revision and Tracking Schedule Document Title Boulders Wind Energy Facility, Western Cape Client Name & Vredenburg Windfarm (Pty) Ltd. Address Document Reference DEA Ref: 14/12/16/3/3/2/1057 Status DRAFT Environmental Impact Assessment Report Issue Date May 2019 Lead Author Dr Alan Carter CES Reviewer Dr Ted Avis CES Study Leader or Registered Dr Alan Carter Environmental Assessment CES Dr Ted Avis Practitioner Approval Circulated to No. of hard No. electronic copies copies Department of Environmental 2 2 Report Distribution Affairs (DEA) Western Cape Government Environmental Affairs And 1 Development Planning This document has been prepared in accordance with the scope of CES appointment and contains intellectual property and proprietary information that is protected by copyright in favour of CES. -

Second Generation Coastal Management Programme 2019 - 2024
WEST COAST DISTRICT MUNICIPALITY SECOND GENERATION COASTAL MANAGEMENT PROGRAMME 2019 - 2024 This Report should be cited as follows: West Coast District Municipality (2019). Draft Coastal Management Programme: Second Generation. This report was developed for the West Coast District Municipality by: The Point, Suite 408, 4th Floor Contact: 76 Regent Road, Sea Point Dr Ted Avis CAPE TOWN Tel: 021 045 0900 8060 Email: [email protected] www.cesnet.co.za Also in Grahamstown, Port Amy Lindsay Elizabeth, East London, Tel: 083 554 9229 Johannesburg & Maputo Email: [email protected] West Coast District Municipality: Charles Malherbe Nick de Jongh CES: Ted Avis Amy Lindsay Acknowledgements: Bergrivier Municipality Cederberg Municipality Matzikama Municipality Saldanha Bay Municipality Swartland Municipality TABLE OF CONTENTS EXECUTIVE SUMMARY ................................................................................................................ VIII 1 INTRODUCTION ...................................................................................................................... 1 1.1 Background ........................................................................................................................ 1 1.2 Defining the Coastal Zone ............................................................................................... 1 1.3 Value of the coast ............................................................................................................. 5 1.4 Context of Coastal Management Programmes in South Africa ............................... -

Water – 2018 Market Intelligence Report –
Water – 2018 Market Intelligence Report – GreenCape GreenCape is a non-profit organisation that drives the widespread adoption of economically viable green economy solutions from the Western Cape. We work with businesses, investors, academia and government to help unlock the investment and employment potential of green technologies and services, and to support a transition to a resilient green economy. Acknowledgments We thank Jane Reddick (lead author) and Bridget Fundikwa (contributor) for the time and effort that went into compiling this market intelligence report. We also thank Raymond Siebrits, Helen Seyler and Jonny Harris for their feedback on draft versions of the report. Disclaimer While every attempt was made to ensure that the information published in this report is accurate, no responsibility is accepted for any loss or damage to any person or entity relying on any of the information contained in this report. Copyright © GreenCape 2018 This document may be downloaded at no charge from www.greencape.co.za. All rights reserved. Subscribe to receive e-mail alerts or GreenCape news, events and publications by registering as a member on our website: www.greencape.co.za. Image courtesy of: Bruce Sutherland 18 Roeland Street, Cape Town, 8001, South Africa Editorial and review: Lauren Basson, Salomé Bronkhorst, Nicholas Fordyce and Claire Pengelly Images: GreenCape, Western Cape Department of Agriculture and Bruce Sutherland Layout and design: Deep Agency II Water: Market Intelligence Report 2018 Water: Market Intelligence Report 2018 III Contents List of figures Executive summary 1 Figure 1 What’s new? 2 Opportunities to reduce reliance on traditional surface water supply 3 1. -

Limonium Dagmarae (Plumbaginaceae), a New Species from Namaqualand Coast, South Africa
Phytotaxa 403 (2): 071–085 ISSN 1179-3155 (print edition) https://www.mapress.com/j/pt/ PHYTOTAXA Copyright © 2019 Magnolia Press Article ISSN 1179-3163 (online edition) https://doi.org/10.11646/phytotaxa.403.2.1 Limonium dagmarae (Plumbaginaceae), a new species from Namaqualand coast, South Africa LADISLAV MUCINA1,2 & TIMOTHY A. HAMMER3 1Harry Butler Institute, Murdoch University, 90 South Street, Murdoch, WA 6150, Perth, Australia; E- mail: Ladislav.Mucina@murdoch. edu.au 2Department of Geography and Environmental Studies, Stellenbosch University, Private Bag X1, Matieland 7602, Stellenbosch, South Africa 3School of Biological Sciences, The University of Western Australia, 35 Stirling Highway, Crawley, WA 6009, Perth, Australia Abstract A new species of Limonium from Namaqualand coast, South Africa is here described and named L. dagmarae. Shared morphological characters with related South African species place the new species within L. sect. Circinaria. Evidence is presented to segregate the new species from relatives based on discrete morphological and environmental characters. The holotype of the L. dagmarae is preserved at NBG. Key words: Afrolimon, Limonium sect. Circinaria, species nova, strandveld, Succulent Karoo Introduction Plumbaginaceae Juss. is a moderately-sized family in the Caryophyllales Bercht. & J.Presl and comprises 25– 30 genera and 650–1000 species (Hernández-Ledesma et al. 2015, Christenhusz & Byng 2016). Limonium Miller (1754: n.p.) is the largest genus in the family with c. 350 species, which are mainly coastal -

Growth Potential of Towns in the Western Cape (2004)
Growth Potential of Towns in the Western Cape (2004) A research study undertaken fot the Department of Environmental Affairs and Development Planning of the Western Cape Provincial Government by: CENTRE FOR GEOGRAPHICAL ANALYSIS UNIVERSITY OF STELLENBOSCH PROJECT LEADER: Prof IJ van der Merwe RESEARCH TEAM: Ms AJ Davids (University of Stellenbosch) Dr S Ferreira (University of Stellenbosch) Mr GP Swart (Urban Dynamics Western Cape) Prof HL Zietsman (University of Stellenbosch) i FOREWORD Linked to the Western Cape’s vision of a ‘Home for All’ and the iKapa Elilhumayo strategy for growing and developing the Western Cape, my Department’s role is to create a Sustainable Home For All – Now and Forever. Drafting the Western Cape Provincial Spatial Development Framework (WCPSDF) has been our major contribution to the iKapa strategies. This Framework is aligned to the National Spatial Development Perspective (NSDP) published by the President’s Office in 2003, which suggests inter alia that Provinces determine the potential for economic growth and human need in towns in the Provinces. Where potential for economic growth is found infrastructure investment is required and where human need is great but economic potential low, social investment in enabling people to participate in alternative labour markets, is essential. Thus it became necessary to understand the potential and challenges of the Western Cape which led us to undertake a scientific study into the Growth Potential of Towns in the Western Cape. During March 2004 the Centre for Geographic Analysis at the University of Stellenbosch was appointed for this task. The information in this document is the result of thorough and careful analysis of the towns outside the City of Cape Town. -

1 July 2019 Prepared for & Five Local
INTEGRATED SECOND GENERATION OF THE WEST COAST DISTSRIC MUNICIPALITY INCLUSIVE OF FIVE LOCAL MUNICIPALITIES AIR QUALITY MANAGEMENT PLAN, 2019-2024 July 2019 Prepared for & Five local municipalities Compiled by: Liketso Tsotetsi Reviewed by: Dr Brett Kuyper 1 EXECUTIVE SUMMARY INTRODUCTION Air pollution is a growing concern across South Africa as a result of ongoing industrialization. The continuous increase of industrial development is important for economic growth, however emissions can be harmful to human health and the environment. It is therefore necessary to regulate industrial emissions that affect air quality. The National Environmental Management: Air Quality Act (39) of 2004 and Air Quality Management Plans (AQMP) are the primary tools used by National and Provincial Government and Municipalities to regulate air quality. There is growing understanding of the relationship between the air quality and climate change highlighting the importance of interlinking the two issues in terms of management in order to achieve clean and safe environment. The developmental framework used in the second generation AQMP was based on the Department of Environmental Affairs (DEA) Manual for Air Quality Management Planning (2012). The AQMP considered the vision and mission statements of the West Coast District Municipality (WCDM) LEGISLATION The National Environmental Management: Air Quality Act 39 of 2004 (NEM: AQA) replaced the repealed Atmospheric Pollution Prevention Act 45 of 1965 (APPA) in South Africa. National government has implemented NEM: AQA through a number of regulations and policies. This second generation AQMP aims to meet these national policies. Furthermore, the AQMP makes provision for climate change mitigation measures and adaptive strategies as part of inherent strategies and intervention plans.